COVID-19 with a prolonged course associated with a state of immunosuppression. Response to treatment with remdesivir
López-Elizondo, Carlos1
López-Elizondo, Carlos1
ABSTRACT
The pandemic due to the coronavirus disease 2019 (COVID-19) has had a variable presentation in the population, from mild to severe cases where large hospital supplies are required for its care. In the case of immunosuppressed patients this disease has had an uncertain behavior in terms of its presentation as well as its prognosis. This clinical case shows a presentation of a prolonged course of COVID-19 associated with a state of immunosuppression due to maintenance treatment with rituximab due to the diagnosis of follicular lymphoma, where a course of migratory pneumonia without the development of acute respiratory distress syndrome is observed (ARDS) being that unlike what has been published in the literature a control and maintenance of the disease is shown only with the use of remdesivir, however the prognosis of the patient is conclusively unknown.KEYWORDS
Coronavirus disease 2019, remdesivir, follicular lymphoma, rituximab, immunocompromised.Introduction
Most patients with COVID-19 present with mild to moderate symptoms, while a minority develop a more severe course of illness that may include complications such as ARDS, septic shock, cardiac injury, and thrombosis.1,2 Immunosuppressed patients with hematologic malignancies have historically been more susceptible to viral respiratory illnesses, including less virulent strains of coronaviruses.3 In general, immunosuppressed patients have been found to be prone to prolonged courses of disease (COVID-19 recurrence), but at the same time may be protected from severe course disease possibly by avoiding the hyperinflammatory state (cytokine storm); however, further information on the component of immunosuppression in this setting is lacking to date.4-6
The elimination of CD20-positive mature B lymphocytes committed to differentiate into autoantibody-producing plasma cells is considered the main effect of rituximab; hypogammaglobulinemia has been reported after rituximab treatment in patients with lymphoma and rheumatoid arthritis; low immunoglobulin G (IgG) has attracted the most attention because of its significant role in protective immunity; however, the incidence and clinical implications of low immunoglobulin M (IgM) have not been studied in detail (the dose, frequency, and number of drug infusions appear to be important variables in this regard). In patients treated with rituximab, B-cell recovery begins at six months of treatment and normal levels are generally recovered within 12 months after the end of treatment, although in some patients it may take longer (up to a median recovery time of 23 months after induction therapy).7,8 The nucleotide analog remdesivir is an antiviral agent approved for the treatment of COVID-19, helping to improve the clinical outcome of patients hospitalized on a five-day regimen.9,10
Here, we report a prolonged course of COVID-19 with SARS-CoV-2 viremia in a patient who received CD20+ lymphocyte depletion therapy via rituximab as maintenance management for follicular lymphoma prior to viral infection, after subsequent remdesivir therapy for five days this patient now shows sustained virologic control of COVID-19.
Case description
This is a 61-year-old man who presented with the diagnosis of follicular non-Hodgkin's lymphoma immunophenotype B (CD20 positive) in 2017, receiving rituximab-cyclophosphamide/doxorubicin/vincristine/prednisone (R-CHOP) treatment being refractory to said scheme, Therefore, he received lenalidomine as well as 18 sessions of radiotherapy with adequate response to such treatment, while rituximab was administered bimonthly (as maintenance) since the end of the R-CHOP scheme (a total of 18 infusions).
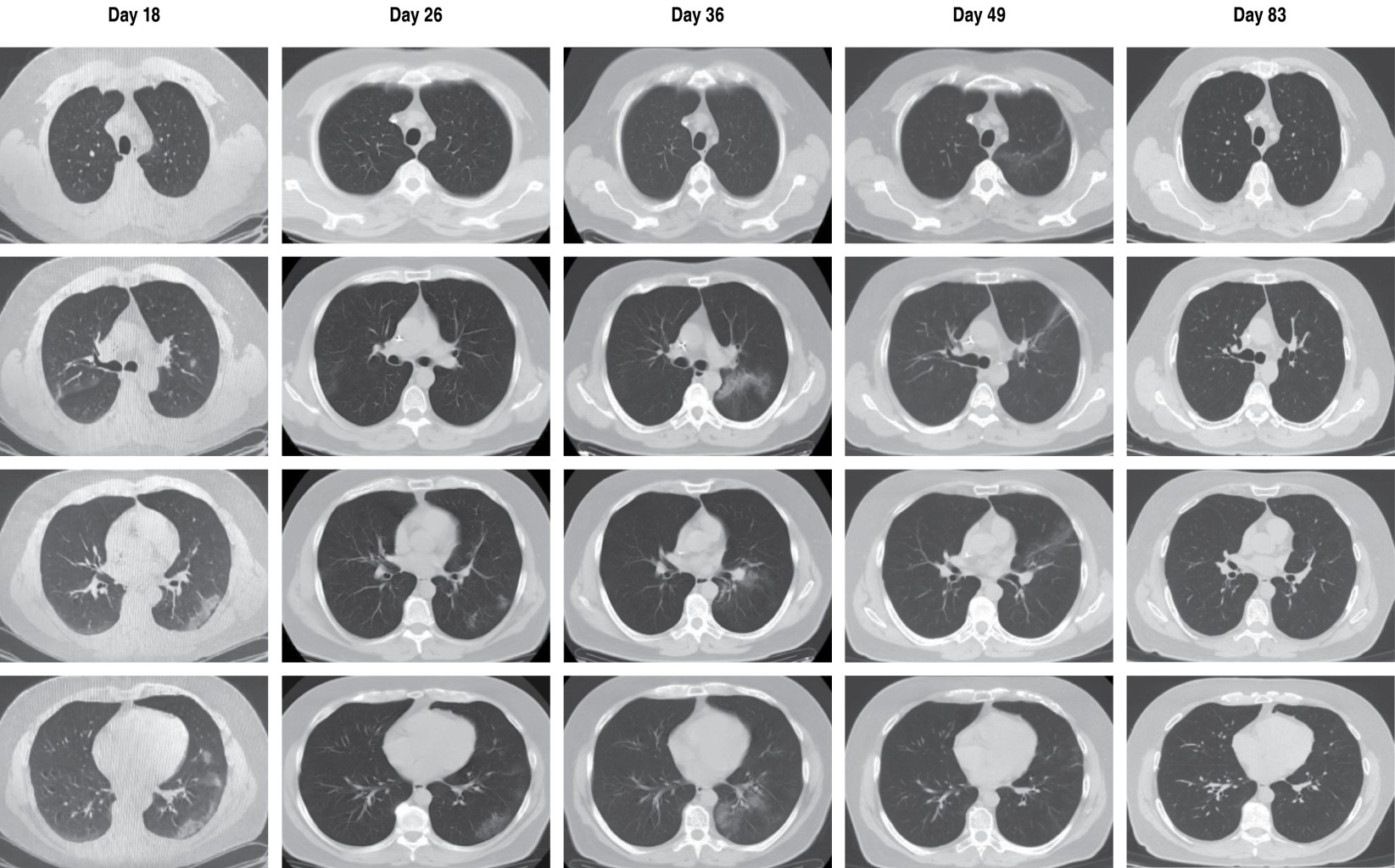
COVID-19 started mildly in January 2021, with improvement only with symptomatic treatment. However, two weeks later the patient begins with febrile peaks without showing hypoxemia by pulse oximetry, and serum labs show lymphopenia and elevated inflammatory markers (Table 1), so a chest CT scan is indicated, which showed areas of ground glass in the right upper lobe and left lower lobe (Figure 1), and symptomatic treatment was started again, but this time adding azithromycin and high doses of inhaled steroids without the need for supplemental oxygen; The patient showed clinical improvement with stabilization of temperature and significant improvement of the areas of ground glass mentioned above (Figure 1), decreasing inflammation at biochemical level and without lymphopenia (Table 1) and with a new negative PCR test for SARS-CoV-2.
One week later, the patient began to have new febrile peaks above 38 oC, as well as a dry cough while maintaining adequate oxygenation, so we proceeded to perform a new chest CT scan that showed a new increase in ground glass and the presence of an alveolar consolidation zone in the left lower lobe (Figure 1), In this context, a new PCR for SARS-CoV-2 was considered, which was positive again, accompanied by elevation of inflammatory markers (Table 1), and the possibility of a reinfection or persistence due to COVID-19 was considered, ruling out other infectious etiologies. In view of the above, the patient was managed without the need for supplemental oxygen with prophylactic anticoagulation (apixaban) and the use of systemic steroids (prednisone), with the empirical addition of levofloxacin and fluconazole; The immunological profile was evaluated, showing decreased total IgM immunoglobulin in serum, as well as lymphocyte subpopulations without detecting CD19+ B lymphocytes by flow cytometry; a quantitative test of specific antibodies for SARS-CoV-2 was also performed, showing an IgG index of 4. 33 (positive) and IgM with 0.74 (negative). Taking into account this immunosuppression status together with the absence of hypoxemia in the patient, it was decided to suspend systemic steroid on the third day of its initiation and remdesivir was started with a scheme suggested in the context of the patient without hypoxemia with 200 mg on the first day, followed by 100 mg once a day for four days intravenously, with which significant clinical improvement was obtained with stabilization of fever and cough 24 hours after starting the medication. The patient remained stable with follow-up at one week with a new PCR test for SARS-CoV-2, which was negative, and also continued with reduction of inflammation at the biochemical level (Table 1).
Finally, the patient remained without new febrile peaks and with improvement of cough, in the follow-up at one month he was practically asymptomatic with control chest CT scan showing almost total improvement of the areas of pulmonary involvement described above (Figure 1), negative PCR test for SARS-CoV-2 and with improvement of inflammation in control laboratories (Table 1).
Discussion
SARS-CoV-2 infection has been shown to trigger an immune response; cases diagnosed with follicular lymphoma have had complete remission of follicular lymphoma following SARS-CoV-2 infection.11 Unfortunately, in other patients with follicular lymphoma with SARS-CoV-2 infection who have previously received chemotherapy treatment, mainly in those who have had controlled disease with maintenance B-cell depletion therapy (anti-CD20 therapy), uncertainty persists about the strength of viral control, the degree of immunity, and the risk of reinfection and/or persistence (reactivation) of viral disease.4,12 The case presented shows a behavior of persistence or reactivation of the viral load manifesting itself with moderate to severe clinical condition (even presenting pneumonia), it is important to mention that at no time had the need for supplemental oxygen, this may speak of a possible protective factor to severe disease by the same immunosuppression that the patient presents but that it does not allow eliminating the viral load.5,13 Contradictorily to the above, there are other studies that mention greater severity and susceptibility of the disease in this population.14
On the other hand, during the evolution of the disease the patient had remained in strict home isolation, so the possibility of a reinfection was considered very unlikely and it was considered as persistent or prolonged course COVID-19, similar to that published in the literature.8 We should also comment on the atypical migratory character of the ground glass lesions that the patient presented at tomographic level (crescentic-menguing effect), ruling out other bacterial, tuberculous and/or fungal etiologies (Figure 1), data similar to that shown in previous publications.4
Regarding the treatment that can be offered for this type of cases, there are few publications and with a low level of evidence, only supported by case reports where plasma from COVID-19 convalescents has been used alone or combined with the use of remdesivir.8,13 In this case, viral control was achieved only with the use of remdesivir, unfortunately regarding this treatment it is unknown whether a sustained viral response can be produced and at what time it can be achieved after the elimination of the virus.
In the PRIMA study, both viral and general infections were found to be increased in patients with follicular lymphoma undergoing rituximab maintenance therapy, the association of increased infections and rituximab therapy is a well-established concept.15
Conclusion
To date, the impact of immunosuppression due to treatment with CD20+ lymphocyte depletion therapy in hematologic malignancies during SARS-CoV-2 disease is unclear, and the extent to which these patients are protected from reinfection by their immune system is unknown. Therefore, the presentation of COVID-19 in this population requires further research to know the prognosis, as well as the best treatment in these patients, pointing out the importance of initiating and/or continuing with the use of such anti-CD20+ therapy in this population with COVID-19 based on the risk-benefit of the same.
AFILIACIONES
1Unidad Médica de Alta Especialidad del Centro Médico Nacional "Ignacio García Téllez", IMSS. Merida, Yucatan. Mexico.Conflict of interests: The author declares no conflict of interests.
REFERENCES
Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42-51. Doi: 10.1016/S0140-6736(10)62175-7
|
Table 1: Evolution at the serum laboratory level. The behavior of the main inflammatory markers for COVID-19 is shown, with ferritin being the most clinically useful. Evolution is shown as the number of days after initial symptoms. |
|||||||
|
|
Day 14 |
Day 18 |
Day 26 |
Day 39 |
Day 49 |
Day 56 |
Day 83 |
|
LHD (U/L) |
337 |
304 |
287 |
327 |
266 |
307 |
318 |
|
ESR (mm/h) |
45 |
42 |
48 |
53 |
72 |
60 |
20 |
|
CRP (mg/dL) |
39.5 |
48.4 |
14.5 |
13 |
2.96 |
2.14 |
0.1 |
|
Ferritin (ng/mL) |
240 |
613 |
319 |
831 |
1,369 |
712 |
468 |
|
D-dimer (µg/mL) |
250* |
0.57 |
508.4* |
0.27 |
0.38 |
0.3 |
0.17 |
|
Fibrinogen (mg/dL) |
--- |
675 |
202 |
488 |
--- |
--- |
--- |
|
Leukocytes (µL) |
6,400 |
5,430 |
4,700 |
5,240 |
5,300 |
6,040 |
4,060 |
|
Lymphocytes (µL), [%] |
1,408 [22] |
850 [15.7] |
1,175 [25] |
650 [12.4] |
940 [17.7] |
860 [14.2] |
990 [24.4] |
|
* (ng UEF/mL) LHD = lactate dehydrogenase, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein. |
|||||||


