Cytomegalovirus and COVID-19 co-infection: case report
Salvador-Ibarra, Ibzan Jahzeel1; Alva-Arroyo, Nancy Verónica1; Pizaña-Dávila, Alejandro1; López-González, Berenice1
Salvador-Ibarra, Ibzan Jahzeel1; Alva-Arroyo, Nancy Verónica1; Pizaña-Dávila, Alejandro1; López-González, Berenice1
ABSTRACT
5% of patients with severe acute respiratory syndrome (SARS-CoV-2) by coronavirus 2 disease (COVID-19) develop acute respiratory distress syndrome (ARDS) resulting in a high mortality rate. A 36-year-old male patient with a history of renal transplant from a related living donor presented with fever of 39 oC, asthenia, adynamia, myalgias and arthralgias. Polymerase chain reaction (PCR) for (COVID-19) was performed, as well as computerized axial tomography (CAT) of the thorax with a finding of CO-RADS 5, he developed greater respiratory insufficiency requiring invasive mechanical ventilation, cultures were obtained with the result of quantitative PCR/DNAc cytomegalovirus (CMV): 554 copies/mL, valganciclovir 900 mg was started, with the patient presenting adequate evolution until mechanical ventilation was withdrawn. Co-infection by CMV and SARS-CoV-2 at pulmonary level should be clinically suspected in the context of pneumonia in the immucompromised patient, favoring the correct and timely treatment that allows complete recovery of the patient.KEYWORDS
Kidney transplantation, cytomegalovirus, COVID-19.Introduction
Over the past year it has been reported that 40-50% of severe acute respiratory syndrome type 2 coronavirus (SARS-CoV-2) infections are asymptomatic;1,2 those who develop symptoms usually have mild to moderate disease, about 15% develop severe pneumonia requiring hospitalization, and approximately 5% develop septic shock and multiorgan failure resulting in a high mortality rate.3,4
Several studies suggest that the course of coronavirus disease 2019 (COVID-19) may be less favorable in immunosuppressed patients. A highly vulnerable group appears to be those with renal transplantation, in whom a mortality rate ranging from 20-28% has been reported,5-9 it has also been proposed that some immunosuppressive therapies such as rituximab are associated with severe COVID-19 when compared with other therapies.
We present the clinical case of a man with a renal transplant who, after showing humoral rejection, was treated with rituximab, in whom, in addition to identifying the SARS-CoV-2 virus, co-infection with cytomegalovirus (CMV) was confirmed, one of the most frequent opportunistic viral pathogens in kidney transplantation. We consider that the success in the treatment of this case lies in the clinical suspicion and identification of the second agent in bronchioloalveolar lavage.
Clinical case
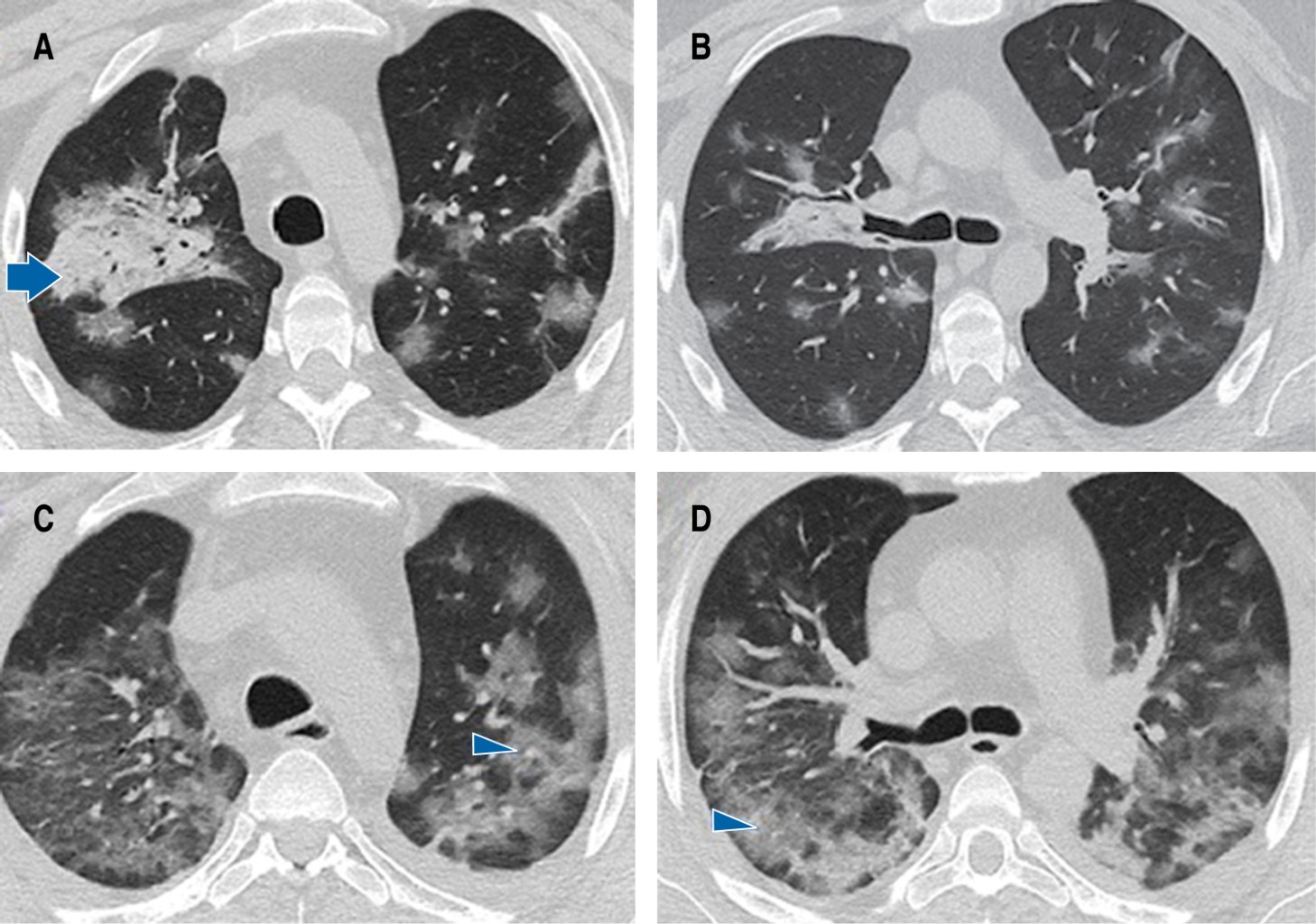
A 36-year-old man with a history of related living donor renal transplantation (2003) for bilateral renal hypoplasia presented humoral rejection in November 2021 and required immunosuppressive management with rituximab, to later receive mycophenolate, prednisone and tacrolimus, conditioning hematologic toxicity and severe immunosuppression. He also suffers from secondary systemic arterial hypertension, in treatment with calcium antagonist and angiotensin II converting enzyme inhibitor. He was admitted for a clinical picture of four days of evolution characterized by fever of 39 oC, dysgeusia, anosmia, cough, myalgias and arthralgias, accompanied by progressive dyspnea up to modified Medical Research Council (mMRC) 4 and pulse oximetry saturation (SpO2) of 88%. On admission to the emergency room he had blood pressure: 105/65 mmHg, heart rate: 85 bpm, respiratory rate: 29 rpm, temperature: 36.7 oC, SpO2: 87%, arterial blood gas (ABG) arterial oxygen pressure (PaO2): 57.6 mmHg, arterial carbon dioxide pressure (PaCO2): 36.6 mmHg, pH: 7.43, arterial oxygen pressure/inspired oxygen fraction index (PaO2/FiO2): 274 mmHg. Chest computed tomography scan confirmed the presence of ground glass areas of random distribution both peripherally and centrally affecting more than 60% of the lung parenchyma (Figure 1A and 1B), and also revealed pancytopenia. Polymerase chain reaction retrotranscriptase (PCR-RT) test for SARS-CoV-2 in nasopharyngeal exudate was positive. Supportive treatment, dexamethasone 6 mg every 24 hours and prophylactic anticoagulation with enoxaparin was initiated. However, 24 hours after hospital admission he developed severe acute respiratory failure syndrome (PaO2/FiO2: 49.4 mmHg) and septic shock. A second simple chest computed tomography scan was performed, which showed significant extension of the pneumonia (Figures 1C, 1Dand 2), so he was admitted to the intensive care unit (ICU) for advanced airway management and assisted mechanical ventilation. Due to clinical and radiological deterioration and because he was a patient with hematologic toxicity due to immunosuppressants, bronchioloalveolar lavage was performed and CMV was identified by polymerase chain reaction/ complementary deoxyribonucleic acid (PCR/cDNA) for cytomegalovirus: 554 copies/mL. The patient's clinical course was torpid, requiring vasoactive amines and renal function replacement therapy. However, after the administration of valganciclovir 900 mg every 24 hours for 21 days, together with the rest of the treatment, the evolution was favorable. Percutaneous tracheostomy was performed due to muscle weakness and prolonged mechanical ventilation; mechanical ventilation was withdrawn after 26 days of stay in ICU, and renal function was recovered, which allowed suspending renal function replacement therapy. The patient was discharged from the hospital five days later with supplemental oxygen 2 L/minute and physical and respiratory rehabilitation. Swallowing test and endoscopic revision of the airway were performed 10 days after discharge, in which malacia and stenosis were ruled out, and the tracheostomy tube was removed.
Discussion
Cytomegalovirus (CMV) is a human virus of the family Herpesviridae, a β-Herpesvirus (HHV) like HHV-6 and HHV-7.10 First infection occurs in childhood and the seroprevalence is 70-90% of the adult population.11 After the first infection, the presence of the virus can be identified in subpopulations of CD34+ myeloid progenitors as well as in CD14+ monocytes, dendritic cells and megakaryocytes.12,13 In situations of immunosuppression, such as solid organ transplantation, CMV infection can occur as a primoinfection or as a reactivation after a long latency period.13,14
In the case presented, diagnosis and follow-up of cytomegalovirus infection was performed with PCR/cDNA testing of bronchioloalveolar lavage specimen, which is the gold standard, and treatment with 900 mg of valganciclovir every 24 hours (O) prophylactically was decided. At present, there is little information on CMV and SARS-CoV-2 coinfection. Amaral et al reported a case of SARS-CoV-2 infection and invasive CMV colitis successfully treated.15
CMV co-infection in patients with COVID-19 increases morbidity and mortality, as CMV reactivation can occur at any time, but is much more likely in the context of graft rejection, severe immunosuppression secondary to graft treatment, with special interest in the use of rituximab, in addition to the patient's critical condition already established by SARS-CoV-2 infection.
Conclusion
To our knowledge, this is the second described case of this co-infection at pulmonary level, so we report our experience, where the co-infection by CMV and SARS-CoV-2 at pulmonary level could be performed thanks to the high clinical suspicion in the context of pneumonia of the immucompromised patient with a history of humoral rejection, in whom the decision to perform bronchioloalveolar lavage allowed a complete etiological diagnosis, facilitating the correct and timely treatment that made possible the complete recovery of the patient.
AFILIACIONES
1Hospital Ángeles Mocel. Mexico City, Mexico.Conflict of interests: The authors declare that they have no conflict of interests.
REFERENCES
Helantera I, Koskinen P, Tornroth T, Loginov R, Gronhagen-Riska C, Lautenschlager I. The impact of cytomegalovirus infections and acute rejection episodes on the development of vascular changes in 6-month protocol biopsy specimens of cadaveric kidney allograft recipients. Transplantation. 2003;75(11):1858-1864. Doi: 10.1097/01.TP.0000064709.20841.E1