Percutaneous embolization of the thoracic duct in iatrogenic chilotorax. Case report
Guerrero-Ixtlahuac, Jorge1; Solano-Velásquez, Melissa Pamela1; Murrieta-Peralta, Estefanía1; Villegas-Villa, Gustavo Adolfo1
Guerrero-Ixtlahuac, Jorge1; Solano-Velásquez, Melissa Pamela1; Murrieta-Peralta, Estefanía1; Villegas-Villa, Gustavo Adolfo1
ABSTRACT
Thoracic duct injury is a rare complication of any intrathoracic surgical intervention but potentially severe if proper treatment is not instituted. Early surgical intervention is required in cases with large refractory chyle output but may be associated with substantial morbidity and mortality. Next, we present the case of a 66-year-old male patient with a history of having undergone radical hybrid esophagectomy for neoplasia in the lower third of the oesophagus, who in the mediate postoperative period presented bilateral pleural effusion, compatible with chylothorax. Treatment was initially conservative; given the unfavourable evolution, it was subsequently treated with percutaneous embolization of the thoracic duct, yielding an adequate resolution. This case demonstrates the efficacy of percutaneous thoracic duct embolization as a treatment alternative, as it is a minimally invasive, safe and effective method.KEYWORDS
chylotorax, thoracic duct, lymphography, embolization.Introduction
Chylothorax is defined as the presence of lymph in the pleural cavity as a result of disruption or obstruction of the thoracic duct. The etiology can be traumatic and non-traumatic, traumatic can be subdivided into iatrogenic and non-iatrogenic.1 Iatrogenic post-traumatic chylothorax remains a major complication after thoracic surgery and particularly difficult to manage.2 Thoracic duct injury is an infrequent complication of any intrathoracic surgical intervention, observed in up to 4%2,3 of thoracic esophagectomies and less than 1%4 in other types of surgery, but potentially very serious, with a mortality of up to 50%5 if adequate treatment is not established.
Lymphatic leakage can occur anywhere along the lymphatic pathway beginning in the four extremities. The most clinically important pathway begins from the intestinal lymphatic ducts and continues through the chyle cistern into the thoracic duct. Chylothorax is a serious condition that can rapidly lead to hypovolemia, electrolyte abnormalities, malnutrition and immunocompromise.6 As a result, early intervention has become the ideal treatment strategy.
In 1998, a new percutaneous method for the treatment of chylothorax was reported by Cope,7 as well as some larger series3 since that date. Percutaneous thoracic duct embolization is a minimally invasive technique with low morbidity and mortality rates and a cure or response rate of up to 73.8% in patients with nontraumatic etiologies and 71% in patients with posttraumatic etiology;2,3 so it is accepted as a therapeutic alternative to surgical treatment in cases in which the fistula is not controlled conservatively.3 However, the procedure is rarely performed. We report a successful case of thoracic duct embolization in iatrogenic chylothorax.
Case report
A 66-year-old man with a history of radical hybrid esophagectomy for neoplasia in the lower third of the esophagus, treated with radiotherapy and neoadjuvant chemotherapy. In the postoperative period he presented bilateral pleural effusion. Pleural drainage tubes were placed through which milky fluid drained; biochemical analysis of the fluid showed high amounts of triglycerides (176 mg/dL), a finding compatible with chylothorax. After conservative treatment, the chest tube debit did not decrease, so it was decided to discuss the case with the interventional radiology service. With the diagnosis of iatrogenic chylothorax, intranodal lymphangiography and embolization of the thoracic duct with coils and cyanoacrylate were performed.
Technique
There are two methods of lymphangiography (LG): bipedal lymphangiography and intranodal lymphangiography. Conventional bipedal LG involves the injection of methylene blue into the subcutaneous cellular tissue between the first and third toes to identify the course of the lymphatic vessels on the dorsum of the foot. Subsequently through a skin incision a lymphatic vessel is isolated to administer Lipiodol®. This method is time consuming and the foot incision can become infected.8,9
Intranodal LG was developed to assess tumor involvement of the inguinal, pelvic and lumbar lymph nodes. Several techniques are described for intranodal LG such as direct puncture of the inguinal nodes blindly or after surgical isolation, as well as ultrasound-guided puncture, which is currently the most widely used,8 as it allows greater precision, is technically simpler, and does not require incisions, thus reducing procedure time, radiation dose and volume of contrast medium.2
Besides identifying the leakage point, LG may have a therapeutic role attributed to the embolizing properties of Lipiodol®, which generates a granulomatous inflammatory reaction during its extravasation and which, due to its viscosity, has the capacity to accumulate at the leakage point as well as in the lymphatic ducts.9
Intranodal lymphangiography
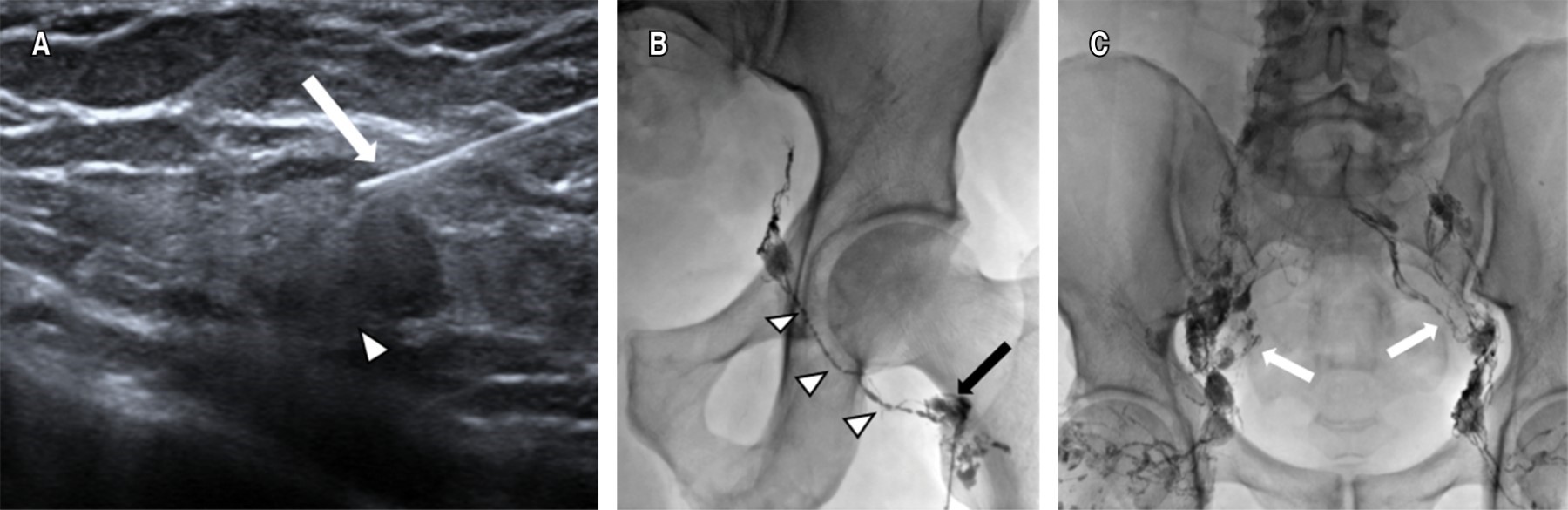
The largest bilateral inguinal nodes distal to the inguinal region were identified under ultrasonographic guidance; Lipiodol® was then injected using a 22 G needle, the tip of the needle being placed in the transition zone between the cortex and hilum of the lymph node. A slow manual injection of Lipiodol® was performed and observed under fluoroscopic guidance in order to confirm the correct position of the needle. A total volume of 10 to 20 mL of Lipiodol® was injected. Images were obtained under fluoroscopic control every five to 10 minutes during the course of the Lipiodol® injection to observe progression through the pelvic and abdominal lymphatic ducts (Figure 1).
Intranodal LG is considered technically satisfactory if the target lymph node is successfully selected and the lymphatic channels of interest, including the chyle cistern, are adequately visualized with Lipiodol®.
Thoracic duct embolization
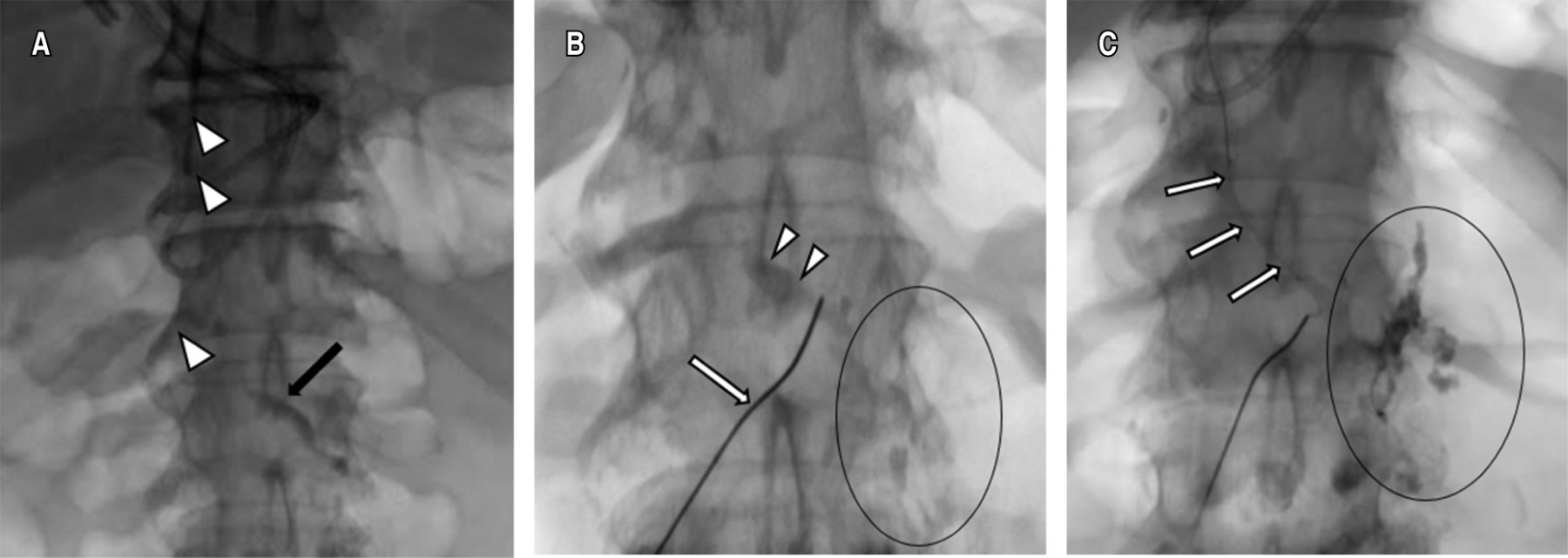
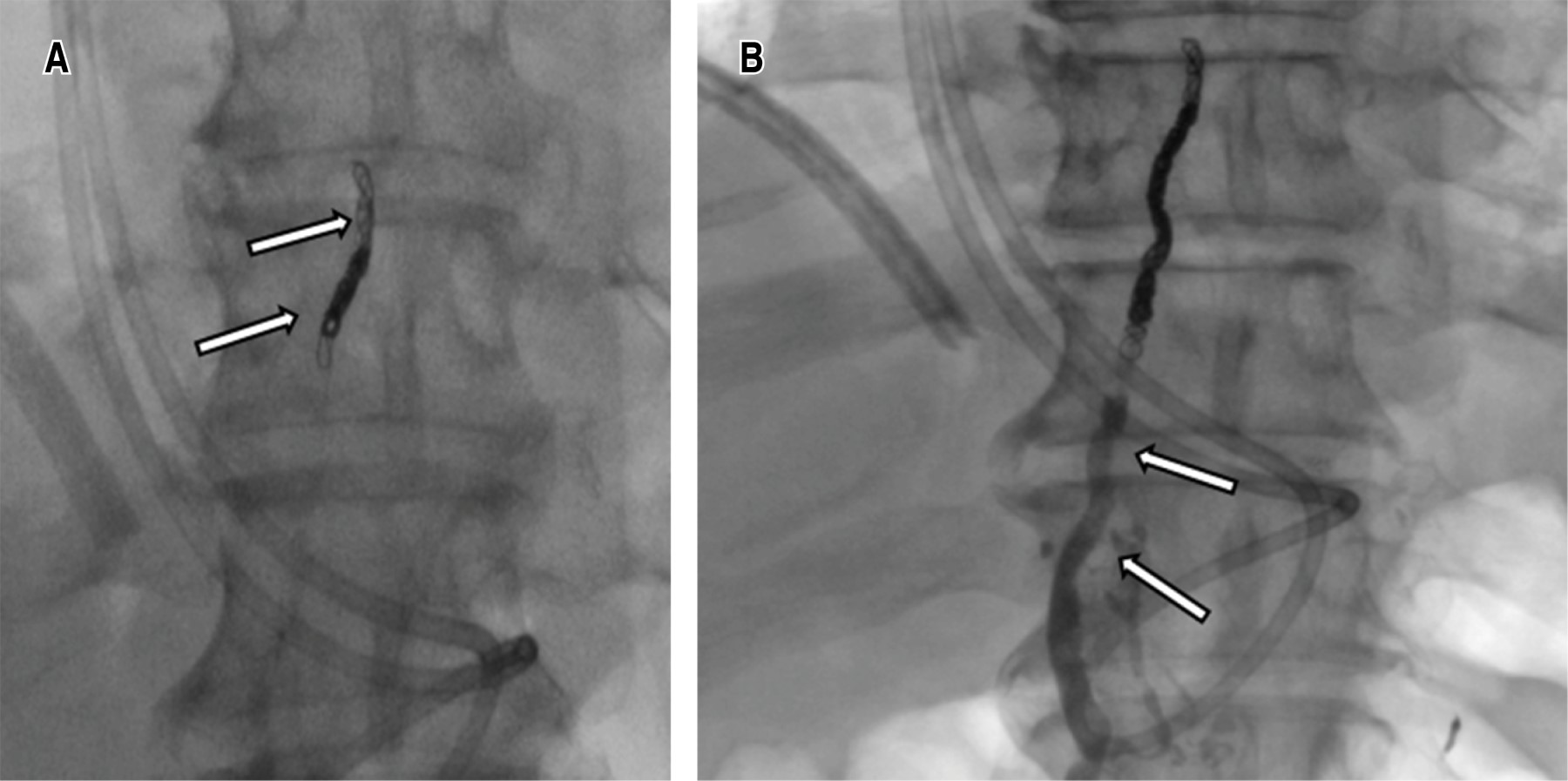
Once the chyle cistern was opacified, a disruption of the thoracic duct and contrast leakage into the left pleural cavity was visualized, under fluroscopic control the thoracic duct was catheterized using a 22 G Chiba needle by percutaneous abdominal approach in the epigastric area and with a slightly cranial angulation. Once accessed, a 0.014-inch guidewire was introduced into the thoracic duct and the needle was exchanged for a microcatheter, which was placed as close as possible to the site of the thoracic duct lesion, ideally joining the point of extravasation (Figure 2). Subsequently, it was embolized with microcoils of 2 and 3 mm in diameter proximal to the leak and the embolization was completed with cyanoacrylate. Control ductography showed the resolution of the leak (Figure 3).
Discussion
Chylothorax is diagnosed by the presence of milky fluid during thoracentesis with a triglyceride content greater than 110 mg/dL and a cholesterol level lower than that of the blood.10,11 Conservative treatment is traditionally used for low-volume chylothorax (< 1,000 mL/dL); however, mortality remains high, up to 50%.5,12 The main causes of surgical traumatic chylothorax include esophagectomy (28%), congenital heart surgery (28%), lung cancer resection (6-27%).13 Overall, esophagectomy is more frequently associated with traumatic chylothorax: 3.9 versus 0.42% in general thoracic surgery.2,3
Thoracic duct embolization was first described by Constantine Cope in 1996 as an alternative to treatment of chylous leaks that were previously not possible without surgical exploration.7 It is recommended when a leak persists for more than two weeks despite conservative treatment, when leakage exceeds 1,000 mL per day for more than five days, or when severe metabolic and nutritional complications occur.1,9 The initial description required pedal LG to opacify the lymphatic system; however, this method is technically challenging and time-consuming, so it has been replaced by ultrasound-guided percutaneous nodal LG.8,14
Thoracic duct embolization has proven efficacy in the treatment of chylous leaks caused by thoracic duct injury. The largest series published in the literature is that of Itkin et al. who evaluated the results of the technique in 109 patients with traumatic chylothorax, reporting a success rate of 71%.2 Pamarthi et al. evaluated the results in 105 patients with traumatic and non-traumatic chylothorax treated by percutaneous techniques, achieving a success rate in traumatic patients of 62%, higher than in patients with non-traumatic chylothorax.15 In the series published by Cope et al.3 where they evaluated the result of percutaneous treatment in 42 patients with chylothorax, they obtained a success rate of 76% in traumatic cases. Therefore, it is considered as a surgical alternative, leaving surgery as the last treatment option in many cases.
In the largest series, no deaths were reported and the complication rate was 3% and included one case of asymptomatic pulmonary artery embolization with cyanoacrylate, two cases of leg edema and foot suture dehiscence.2 This is significantly lower than the mortality of 82.1% and morbidity of 38.3% in surgical cases.4
In our case, a good technical and clinical result was obtained, with no recurrence of the chylothorax, no need for reinterventions and no morbidity. It is in support of the new approach to chylothorax treatment advocated by some other authors,2,3,15 who consider thoracic duct embolization as a first-line option in patients with refractory chylous effusions. Despite superior results, thoracic duct embolization is a technically challenging procedure performed in very few centers due to unfamiliarity with LG.
Conclusions
Lymphangiography remains an important tool in the localization of chylous leaks. It has evolved from a laborious and time-consuming procedure that relied on foot lymphangiography to one in which inguinal intraganglionic lymphangiography shortens time and minimizes risks. With proper case selection, thoracic duct embolization is a minimally invasive alternative to surgery in the treatment of traumatic and nontraumatic cases of chylothorax.
AFILIACIONES
1Instituto Nacional de Cancerología. Mexico City, Mexico.Conflict of interests: the authors declare that they have no conflict of interests.
REFERENCES