Malignant fibrous histiocytoma, case report
Martínez-Osorio, Juan Carlos1; Escalera-Bustamante, Manuel Antonio1; Ortiz-Cordero, Luis1; Escobedo-Sánchez, Emmanuel1
Martínez-Osorio, Juan Carlos1; Escalera-Bustamante, Manuel Antonio1; Ortiz-Cordero, Luis1; Escobedo-Sánchez, Emmanuel1
ABSTRACT
The term malignant fibrous histiocytoma was introduced by O'Brien and Stout for tumors of fibrocystic lineage. Malignant fibrous histiocytoma is the second most common soft tissue sarcoma of the musculoskeletal system after liposarcoma. The treatment of choice is surgery with wide resection margins, with radiotherapy and chemotherapy being complementary treatments to surgery. Study of a 68-year-old female patient in a Thoracic Surgery Service. Patient with malignant fibrous histiocytoma, observation and detailed description of the patient's clinical history, evolution of the disease, treatment and results obtained in the patient. Risk factors, such as exposure to biomass. Multidisciplinary management allows a timely approach and benefit in the result of long-term treatment for the patient. The use of the VAC system and thoracoscopy, to work together in the surgical approach, allow the reduction of wound healing and closure time, together with a reduction in the days of hospital stay, while also reducing hospital costs inherent to these pathologies.KEYWORDS
case report, thorax surgery, plastic surgery, VAC system, malignant fibrous histiocytoma.introduction
Malignant fibrous histiocytoma is the most common soft tissue tumor in adults, the term was first introduced in 1963. O'Brien and Stout introduced the term malignant fibrous histiocytoma for tumors of fibrocystic lineage. Weiss and Enzinger described myxoid malignant fibrous histiocytoma, which shares several features with myxofibrosarcoma; and classified it into grades, according to its histology: low grade (myxoid predominant), intermediate grade (mixed: myxoid and cellular) and high grade (predominantly cellular).1 However, in 2002 the World Health Organization classified malignant fibrous histiocytoma as an entity and determined that the myxoid type without myogenic, lipoblastic and chondrogenic factors is classified as myxofibrosarcoma.2
Malignant fibrous histiocytoma is the second most frequent soft tissue sarcoma of the musculoskeletal system after liposarcoma. It arises from pluripotential mesenchymal cells capable of differentiating from histiocytes, fibroblasts and myofibroblasts found in connective tissue.3
Macroscopically, malignant fibrous histiocytoma appears as a large tumor with multiple areas of necrosis on the cut surface. Microscopically the tumor shows a disorderly proliferation of spindle cells with occasional stellate or swirling pattern, presence of multinucleated cells with large nuclear atypia, bizarre shapes, frequent atypical mitosis figures, and a stroma showing a large amount of collagen, as well as a variable number of mononucleated inflammatory cells and foamy histiocytes. Its diagnosis is clinical and paraclinical. Cruz states that in 74% of cases it is made on the basis of macroscopic and microscopic morphology, but even so he recommends the use of immunohistochemistry, which is considered fundamental by Miettinen because of the heterogeneity of these lesions.4
Multidisciplinary treatment is essential for two reasons: 1) because the prognosis of these neoplasms is determined by the histological grade and size of the tumor; and 2) because up to 22% of cases present clinical metastatic disease from the outset.4
Case presentation
Female patient aged 68 years with primary school education, originally from Guanajuato, occupation: flower trader and exposure to fertilizers for 11 years. Allergies to contrast medium, allergy to drugs: trimethoprim-sulfamethoxazole, omeprazole, gentamicin and metamizole.
Diagnosed with type 2 diabetes mellitus for 11 years, on treatment with metformin 850 mg every 24 hours and insulin lispro 50 IU in the morning and 30 IU at night every 24 hours. History of systemic arterial hypertension in treatment with losartan 50 mg every 12 hours, since 11 years ago. Diabetic neuropathy in treatment with pregabalin 75 mg every 24 hours for the last five years.
Surgical history: a cesarean section 32 years ago without complications, a laparoscopic fundoplication in 2001 secondary to gastroesophageal reflux disease without complications, a total abdominal hysterectomy secondary to uterine myomatosis 15 years ago with need for blood transfusion (type and amount of blood products transfused are unknown). A laparotomy for intestinal occlusion 15 years ago without complications and an open cholecystectomy 13 years ago, without complications.
She started her current condition in November 2021 with pain in the left hemithorax and shoulder, pulsating, with intensity 5/10 VAS (visual analog scale), intermittent, accompanied by weight loss of 5 kg in the last month, fever and nocturnal diaphoresis. She consulted a physician and received multiple treatment with non-steroidal anti-inflammatory drugs (NSAIDs), without improvement.
From day one of hospital stay, she started with antibiotic therapy with ceftriaxone 1 g every eight hours, her control medications metformin, losartan and insulin lispro. On her fourth day analgesia with paracetamol and ketorolac was added, as she began to report pain of intensity 6/10 VAS.
On his fifth day of in-hospital stay he presented dyspnea and hypertensive crisis, enoxaparin 60 mg every 24 hours, nifedipine 30 mg every 24 hours and oxygen with nasal prongs 3 L × min were added to his treatment regimen.
On his seventh day of stay, he underwent radiography of the shoulder and left arm, with no abnormal radiological data, only with increased volume in the soft tissues of the left arm. An axial, coronal and sagittal computerized tomography was also performed, showing a hypodense area at the level of the left costoclavicular joint, and a central venous catheter was placed.
On the tenth day of her stay, she received a consultation from the psychology department, which showed improvement in her mood. On this day she was admitted to the operating room, and on 03/24/2022 a malignant tumor of the thorax was excised. Surgical findings showed an 8 cm diameter tumor in the anterior sternal face and left hemithorax with undefined borders, indurated, fixed to deep planes, with fatty infiltration, erythematous changes and presence of erosion at cutaneous level, with left pleuropulmonary invasion in the upper lobe, very friable tissue of petrous consistency, without invasion of vessels.
Technique: a spindle incision is made at the level of the anterior wall leaving a 4 cm border peripheral to the lesion and block resection is performed with exeresis of the left clavicle, first and second ribs, as well as partial sternotomy of the manubrium and upper third of the sternal body to the cartilage of the first and second right costal arches, as well as the clavicular union, identifying without invasion of the subclavian vein and artery, innominate vein and large vessels. Non-anatomic resection of the anterior wall of the upper pulmonary lobe infiltrated by tumor was performed with a stapler, with no evidence of leakage (Figure 1A). Hemostasis was verified and DualMesh was placed in the pleuropulmonary resection site, as well as StraTos system bar placement in the second costal arch, bilaterally (Figure 1B). White sponge is placed to cover large vessels and then VAC system (vacuum-assisted closure) is placed with gray sponge at a continuous suction of 50 mmHg, left endopleural probe is placed under direct vision with thoracoscope, seal is placed with continuous suction at 20 cm3.
Plastic surgery (25/03/2022): incision on previously marked cutaneous island at the level of the left lumbar region, 19 × 9 cm, spindle-shaped, cutting dissection is performed until reaching the aponeurosis of the latissimus dorsi to its anatomical limits, disinsertion of the latissimus dorsi muscle from lateral, medial and inferior end is performed, disinserting the muscle with superior pedicle up to 3 cm below the axillary hollow. Hemostasis is verified. Biovac drainage is placed in the left lumbar region. A suprafascial tunnel was made at the left axillary level towards the cruciate area in the anterior hemithorax measuring 19 × 9 cm. Wound plane closure was performed on the dorsum, SCT (subcutaneous cellular tissue) with 2-0 vicryl inverted in deep plane, 3-0 vicryl in dermis and skin with staples. BIOVAC type drainage is fixed with 3-0 nylon. The patient is placed in dorsal decubitus and the VAC system is removed, gray sponges and white sponge are removed, showing bone exposure with loss of clavicular segments and first and second ribs resected in the previous procedure with StraTos system bar in bilateral second costal arch, pulmonary exposure with DualMesh, exposure of great vessels, pectoralis major segments and adjacent nerves. We proceed to externalize the previously tunneled flap and fix the wide dorsal flap to the deep tissue, place the cutaneous island in its final position (Figure 1C), place a superolateral Penrose drain and another inferolateral Penrose drain. After hemostasis, closure is performed by planes, 2-0 vicryl inverted stitches for SCT and then 3-0 vicryl inverted stitches, the skin is faced with cardinal staples.
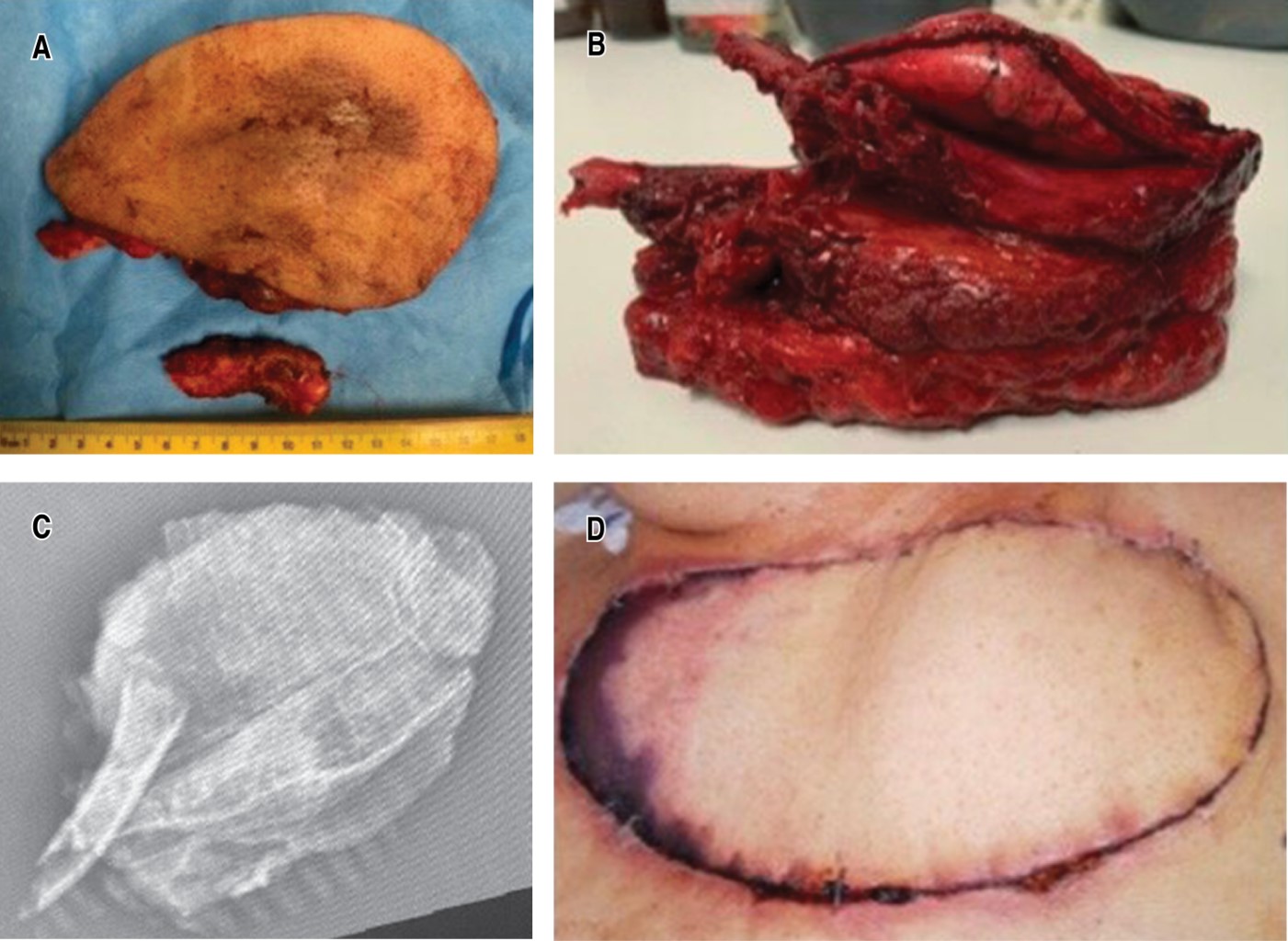
The surgical specimen is shown with respected edges (Figure 2A and B), of which radiography is taken (Figure 2C). Follow-up image is shown two weeks after surgery (Figure 2D).
Discussion
Secondary to a multidisciplinary management between surgical services such as thoracic surgery (resection) and plastic and reconstructive surgery (reconstruction), it was possible to obtain beneficial results and survival for the patient, and the patient has been continuously followed up by both services until today.
Conclusion
Multidisciplinary management is of utmost importance to ensure the patient's well-being. The joint management allowed a timely and beneficial approach in the outcome of long-term treatment for the patient. The use of the VAC system and thoracoscopy to work together in the surgical approach allows a reduction of the healing time and wound closure, a shortening of the days of hospital stay and also a reduction of hospital costs inherent to these pathologies.
Acknowledgments
Prof. Marlene Muzquiz Flores for all her academic support. Miguel Alejandro Martínez Arias, chief of thoracic surgery, CMI; M. Linda Sofía, research physician associate to chief of thoracic surgery, CMI.
AFILIACIONES
1Centro Médico, Instituto de Seguridad Social del Estado de México y Municipios. Toluca, Mexico.Conflict of interests: the authors declare that they have no conflict of interests.
REFERENCES