Recommendations for diagnostic approach and management of bronchiectasis
Hernández-Zenteno, Rafael de Jesús1; Velázquez-Montero, Alejandra1; Suárez-Landa, Teresa de Jesús1; Pérez-Padilla, José Rogelio1
Hernández-Zenteno, Rafael de Jesús1; Velázquez-Montero, Alejandra1; Suárez-Landa, Teresa de Jesús1; Pérez-Padilla, José Rogelio1
ABSTRACT
Bronchiectasis is a syndrome of chronic cough and production of viscous sputum associated with dilation of the airways and thickening of the bronchial wall. Exacerbations are usually caused by bacterial infections. It is a chronic disease that requires rapid responses for the treatment of exacerbations. Bronchial secretions should be cultured. Evaluate and treat underlying diseases to interrupt progression. In patients who have recurrent exacerbations (two to three in the last year) and do not have Pseudomonas aeruginosa infection, preventive therapy with a macrolide is recommended, excluding nontuberculous mycobacterial infections. In patients with recurrent exacerbations, or significant morbidity, and Pseudomonas aeruginosa in sputum, a therapeutic trial of nebulized tobramycin is useful. Nebulized tobramycin may also be for patients not infected with Pseudomonas aeruginosa in whom oral antibiotic prophylaxis is contraindicated, not tolerated, or ineffective. Patients who have Pseudomonas aeruginosa but cannot receive a nebulized antibiotic may benefit from macrolides as an alternative. Inhaled glucocorticoids are only indicated in patients with asthma or COPD. For patients who respond to bronchodilators on spirometry, the use of inhaled beta-adrenergic agents is suggested. All patients are candidates for pulmonary rehabilitation and bronchial hygiene. The prognosis is influenced by the underlying disease process, the frequency of exacerbations, and comorbidities, but in general, age-adjusted mortality is increased compared with the general population.KEYWORDS
bronchiectasis, approach, diagnosis, treatment.Abbreviations:
Introduction
Bronchiectasis is an acquired disorder of bronchi and bronchioles, characterized by a permanent abnormal dilatation and destruction of their walls. Its induction requires an infectious insult plus drainage alteration, airway obstruction or defects in the defenses of the host. Bronchiectasis share many clinical presentations with chronic obstructive pulmonary disease (COPD), including collapsible airway inflammation, expiratory airflow obstruction, frequent exacerbations that require scheduled or unscheduled consultations or hospitalization. Diagnosis based on the clinical history (daily cough, tenacious discharge expectoration, recurrent expectorations and, by imaging, bronchial dilatations).1
Epidemiology, diagnostic approach, pharmacological and non-pharmacological management are the objective of this review that aims to be a proposal for recommendations.
Epidemiology
Prevalence increases with age eight to 10 times after the 60 years of age (300-500/100,000) when compared to < 40-50 years of age (40-50/100,000).2 In United States a prevalence of 350,000-500,000 is estimated in adults.3 Medicare (≥ 65 years of age) has an annual prevalence of 701/100,000 inhabitants.4
The greatest risk factor for chronic cough in the non-smoking population is bronchiectasis (OR = 5), among the former smokers it is OR = 7. It is more common in women, they make an extensive use of health resources (consultations, antibiotics, CT scans and hospitalization),2,4 its prevalence is higher in the marginalized population, affects young people and impacts survival.5,6 Social and environmental factors undoubtedly play a rol, including smoke exposition, limited access to health services and delayed antibiotic prescription.
Mortality: some small studies have described mortality rate of 16-20% at five years, which increase with the hospitalization in the Intensive Care Unit (ICU) and comorbidity. A study in United Kingdom found that mortality adjusted by age for adults with bronchiectasis was approximately twice that of the general population, regardless of age difference.7 A report of 48 patients from France found 19% mortality in the ICU and 40% mortality per year.8 In one series of 57 patients in Singapur a 26% general hospitalization mortality was reported without difference if the patients received non-invasive ventilation or intubation with mechanical ventilation.9 Severe hypoxemia and high APACHE II scores were the worst prognosis factors.
In one series of 245 patients with bronchiectasis in Belgium between 2006 and 2013, mortality was 20%, increasing to 55% among those with COPD.10 The cause of death was mainly respiratory (58%).
Pathophysiology: the consequent host response, immune effector cells (mainly neutrophils), neutrophils proteases (elastase), oxidative stress (hydrogen peroxide, H2O2) and inflammatory cytokines create a transmural inflammation, mucosa edema, ulceration and neovascularization in the airways.11 The following factors may contribute to the physiopathology of bronchiectasis:
Risk factors
History of pneumonia (in childhood), alcoholism (bronchoaspiration, GER), pertussis, measles, tuberculosis (and granulomatosis), asthma, allergies, rheumatism, infertility, inhaled agents.22-24 In Table 1 shows the causes grouped by etiology and their diagnostic approach base on studies.22-24
Implications and complications
Decreased pulmonary function: patients with bronchiectasis have a mean annual decrease in forced expired volume in the first second (FEV1) of 50-55 mL/year.25 This is higher than in normal individuals (20-30 mL/year), but similar to patients with COPD (approximately 60 mL/year). Among patients with bronchiectasis, FEV1 decrease accelerates when there is Pseudomonas colonization, frequent exacerbations, or increased inflammatory markers (e.g., C reactive protein).
Pulmonary vascular disease: an observational study evaluated 94 patients with bronchiectasis by echocardiography.26 There was evidence of pulmonary hypertension (defined as an estimated systolic pulmonary arterial pressure > 40 mmHg) in 33% of patients and right ventricular systolic dysfunction in 13%. Right ventricular dysfunction was correlated with low FEV1, low diffusing capacity for carbon monoxide (DLCO), hypercapnia and hypoxemia. Only 15% of patients had evidence of left ventricular dysfunction.
Hemoptysis: the origin of bleeding in bronchiectasis is due to the rupture of a tortuous bronchial artery or submucosal capillary plexus. It is a frequent and serious complication of the bronchiectasis. Hemoptoics are common in stable patients, while, the occurrence of increased amount of fresh blood or clots during an acute exacerbation is less common; bronchiectasis is a common cause of life-threatening bleeding. The most common causes of hemoptysis in bronchiectasis are mycobacteria and fungi. When bleeding is present, the time, amount and condition of the patient should be evaluated. The approach to bronchiectasis based on hemoptysis escapes the approach of these recommendations.
Cardiovascular morbidity: respiratory tract infections are associated with increased cardiovascular events (CV): myocardial infarction, stroke.27 In a revision of patients with bronchiectasis from primary care practices in the United Kingdom, an increase in CV events was observed in the first 90 days after higher relative risk (RR) respiratory infection in the first three days.28 In a separated study, bronchiectasis was an independent risk factor for the coronary artery disease and strokes after the adjustment for age, sex, smoking and other known CV risk factors.29 Serum desmosine, is a marker of elastin degradation, it may be a marker of CV mortality.30
Classification of severity and prognosis
Few studies have examined the frequency of exacerbations, the hospitalization, comorbidities and mortality, as well as the rate of pulmonary decline function among patients with bronchiectasis; long-term outcome studies are limited.8,25,31 Score systems have been propose to help guide prognosis assessment and identify patients who exacerbate frequently.32,33 The bronchiectasis severity index (BSI) (Table 2), was derived from 608 patients with bronchiectasis in a center in Scotland and was validated in 597 patients in other centers of the United Kingdom and Europe.32 The predictors of hospitalization included prior hospitalization high index of dyspnea, low FEV1, presence of Pseudomonas in the sputum and more extensive involvement (> 3 lobes) in high Resolution CT Scan. Mortality was correlated with advanced age, low FEV1, prior hospitalization and three or more exacerbations in the last year. This score system presented a prognosis capacity for all the causes of mortality at four year of diagnosis, it also presented value for future hospitalization.34
FACED score (Table 3) is an easy scale to use composed by five variables and 10 points (FEV1, Age, presence or not of Colonization/ chronic bronchial infection for Pseudomonas, radiological Extension in the high resolution CT Scan mentioning the number of lobes affected and Dyspnoea measured by the modified Medical Research Council scale [mMRC] dichotomized in 0-II and III-IV, the higher score the more dyspnoea). It was developed in 397 subjects from a multi center cohort of 819 patients from Spain.33 This scale presented an excellent predictive capacity for all causes of mortality five years after diagnosis and for respiratory causes.34
The BSI and FACED were evaluated retrospectively over 19 years with respect to mortality estimates in 91 patients followed at the Royal Brompton Hospital in London. Both scores gave equally mortality estimates at five years, with the FACED slightly higher at 15 years.35 Regarding, other clinical results, in an additional analysis of 1,612 subjects of seven European cohorts, the BSI more accurately predicted exacerbations, hospitalizations, respiratory symptoms, and quality of life than the FACED score.36
Daily sputum production and the presence of Pseudomonas or other potential infectious pathogens in sputum culture were the main characteristics related to quality of life (QoL = quality of life), inflammatory markers and the clinical results at three years.37
Clinical criteria to guide management
Treatment
The goals of bronchiectasis treatment are to prevent exacerbations, reduce symptoms, improve quality of life, and stop disease progression.
The underlying cause should be treated specifically, CFTR (cystic fibrosis transmembrane conductance regulator) modulators in CF; DNase in primary ciliary dyskinesia; antibiotics for non-tuberculous mycobacterial infections; macrolides in diffuse panbronchiolitis; intravenous or subcutaneous immunoglobulins in immunodeficiencies; inhibitors of acid secretion in gastroesophageal reflux (GER); oral and antifungal corticosteroids in allergic broncho pulmonary aspergillosis; smoking withdrawal; intravenous alpha 1 antitrypsin in PIZZ phenotypes, management of associated diseases (COPD, asthma, inflammatory bowel disease, systemic diseases);and surgery or bronchial dilatation in bronchial obstruction.
An exacerbation of bronchiectasis is defined as a deterioration in three or more of the following symptoms: cough, sputum volume or consistency, sputum purulence, shortness of breath or exercise intolerance, fatigue or malaise, hemoptysis lasting at least 48 hours, accompanied by a change in the treatment of bronchiectasis, and exclusion of other possible causes of clinical deterioration.38 It may be accompanied by changes in respiratory examination, deterioration of lung function, or increased markers of inflammation. The pathogens that are most often isolated in an exacerbation are: P. aeruginosa, H. influenzae, S. pneumoniae, S. aureus, Moraxella catarrhalis and enterobacteria.39 Viruses are isolated in 25% of cases (coronavirus, rhinovirus, influenza, SARS-CoV-2).25
Exacerbations
Antibiotic therapy is the cornerstone of treatment because it reduces the bacterial load and systemic and airway inflammatory mediators.40 Ideally, it should initially be adapted to previous sputum cultures and sensitivities, where possible rather than chosen empirically. Other factors in antibiotic selection are the route of administration, oral or parenteral, the history of success or failure, and the presence of allergy or intolerance. Do not use nebulizad antibiotics as single agents in an acute exacerbation.41
Mild exacerbation: most afebrile and clinically stable patients (mild exacerbation) can be treated on an outpatient basis with an oral antibiotic guided by the most recent sputum culture results, and by the patient's experience with previous regimens. In the absence of culture, a respiratory fluoroquinolone (e.g., levofloxacin, moxifloxacin)is a reasonable and broad-spectrum option. In cultures without positive beta-lactamases (H. influenzae or Pseudomonas), the options are amoxicillin or macrolide. It can be modified based on response to therapy and culture results and sputum sensitivity. In beta-lactamase positive culture (M. catarrhalis or H. influenzae) the options are amoxicillin-clavulanate, second or third generation cephalosporin, azithromycin or clarithromycin, doxycycline or a fluoroquinolone.41 In positive culture of P. aeruginosa, the initial selection is ciprofloxacin. Given previous courses of antipseudomonal, resistance to quinolones often requires IV. Due to the propensity of P. aeruginosa, it is recommended to add nebulized tobramycin to ciprofloxacin.42,43
Clinical experience favors a duration of 10-14 days for patients with a first time or few exacerbations. The European Respiratory Society (ERS, 2017) guidelines suggest a 14-day cycle. When there is no response or relapses in a short time, repeat culture them (Table 4).44
Severe exacerbation: when there is increased respiratory rate ≥ 25/minute, hypotension, temperature ≥ 38 °C, hypoxemia (pulse oxygen saturation < 92%) or lack of improvement after oral antibiotics (no intravenous therapy at home), hemoptysis, severe cardiopulmonary instability, or the presence of resistance to available oral agents, initial intravenous and hospital treatment is appropriate.45 Always obtain sputum culture before starting antibiotics. A significant number of severe exacerbations are by Pseudomonas and if they are resistant to oral quinolones, antipseudomonas penicillin can usually be used as ceftazidime; in case the patient looks seriously ill or has incipient Pseudomonas pneumonia a second agent (e.g. fluoroquinolone, aminoglycoside) can be added (Table 5).45
Treatment of severe exacerbation should be 14-21 days; a short treatment of seven days will depend on exacerbation severity, patient conditions, and expectoration cultures.41
Antivirals are indicated when the etiology is due to influenza virus (oseltamivir or oral baloxavir). For SARS-CoV-2 should be individualized based on symptoms, risk factors and severity of disease.
Eradication of P. aeruginosa
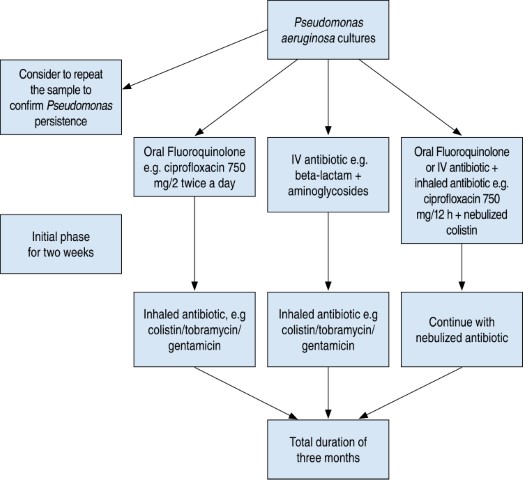
When there is evidence of new isolation and clinical deterioration. Multiple treatments have been suggested. A practical way is as seen in Figure 1.
After completing the eradication treatment, a monthly sputum culture must be performed for the first three months and then every two months for a year. It would be a failure of eradication if a positive culture returned during the first year. We will add a nebulized treatment if it had not been added initially, if it had been added we must repeat the same regimen of ciprofloxacin plus nebulized antibiotic, or change the nebulized treatment used in the first regimen. If at least two strategies with nebulized and oral antibiotics fail, the use of nebulized plus IV treatment is recommended. If at least three strategies fail, it should be considered a chronic infection.46
Treatment of chronic infection
It is defined as having two or more isolates of the same organism at least three months apart in a year.11 The most commonly associated germs are Haemophilus influenzae and P. aeruginosa, less commonly Streptococcus pneumoniae, Staphylococcus aureus, and Moraxella catarrhalis.46,47
Chronic infections, particularly P. aeruginosa, potentiate airway inflammation and are associated with increased frequency of exacerbations, hospitalizations, reduced quality of life, increased mortality, and increased health care costs.
For treatment, the nebulized route is recommended because it reduces exacerbations and decreased lung function, possibly by reducing bacterial load and airway inflammation. It has also been shown to provide consistent antibiotic deposition, high concentrations in ventilated areas of the lung with a lower risk of toxicity or systemic adverse effects versus other routes. A systematic review and meta-analysis of 16 studies, with a treatment duration between four weeks to 12 months demonstrated significant reduction in the number of exacerbations with nebulized treatment. It was well tolerated with low proportions of adverse effects (bronchospasm), which disapeared with drug discontinuation. There was an increase in antimicrobial resistance at the end of the study, but it appeared to decrease after discontinuation. Nebulized antibiotics did not improve quality of life.48
Spanish regulations (Table 6)44 recommend maintaining the nebulized route for long periods of time according to risk/benefit and depending on the selection of the antibiotic with continuous or intermittent guidelines. If with the intermittent form there is clinical worsening, it may be considered to alternate with another nebulized antibiotic without rest periods between them. If poor control persists, oral or intravenous antibiotic therapy should be associated every 1-2 months.
Long-term treatment (prophylactic)
To those who have ≥ 3 exacerbations per year (frequent exacerbators), to prevent exacerbation. Macrolides (azithromycin, erythromycin) are suggested as the first line due to the high quality, evidence in decreasing exacerbations and acceptable side effect profile. In the case of P. aeruginosa infection add a long-term nebulized treatment.
British Thoracic Society (BTS) guidelines suggest for colonization by P. aeruginosa:49
No colonization by P. aeruginosa:49
Muco active and mucolytic treatment
In those who frequently have difficulty in expectorating, or abundant secretions where standard airway cleaning techniques have failed to control symptoms, hypertonic substances and mannitol can be used long-term (≥ 3 months).49
Nebulized hypertonic saline (6-7%) is related to improved mucus clearance, increased ciliary motility, and improved cough clearance. Low mucus salinity contributes to mucus retention. It may improve FEV1 combined with chest physiotherapy and is not superior to 0.9% saline.50,51
Mannitol is a hyperosmolar agent that is believed to hydrate secretions, improving mucus clearance. There is insufficient evidence. In a therapeutic multicenter trial (the largest in bronchiectasis) 461 patients inhaled mannitol dry powder 400 mg or mannitol 50 mg (control) twice daily for 52 weeks, showed only modest significant improvements in time to first exacerbation, antibiotic days and quality of life according to St. George Respiratory Questionnaire (SGRQ). A post hoc analysis of 333 patients showed that the greater the burden of symptoms, the time to first exacerbation and fewer exacerbations against placebo were reduced.52,53
Aerosolized dornase alpha (recombinant deoxyribonuclease, also called DNase), which breaks down DNA (gelatinous product of neutrophils), improves FEV1 and reduces hospitalizations in CF patients,53 but is not effective in another etiology and is potentially harmful.54
Mucolytics, essentially N-acetylcysteine, for one year were associated with a reduction in exacerbations and sputum volume, and also improved quality of life.55
Other medical therapies
Bronchodilators: should be used in patients with asthma or COPD49,56 or with significant dyspnea. Airflow obstruction should be assessed by pre- and post-bronchodilator spirometry. If there is reversibility inspirometry, a trial with short-acting beta agonist (SABA) is almost always initiated.46 If symptoms improve, continue with SABA or prolonged (LABA). If no airway obstruction is demonstrated, they are not indicated. Information is missing in this context.
Oral glucocorticoids (GC): their use is reserved. In other types of patients, it is suggested to avoid this treatment because they can depress the immunity of the host, promote bacterial and fungal colonization, which perpetuates the infection. Only in patients with asthma or allergic bronchopulmonary aspergillosis it may be used.
Inhaled glucocorticoids (ICGs): there is no evidence for their use when they do not coexist with asthma or COPD as a concomitant disease.45,57 The ERS guidelines recommend it only in such a situation.10 They show no significant effect on spirometry, exacerbation rate or sputum volume. Patients with serum eosinophils > 3% improved quality of life (SGRQ) at six months.58 Although its use was associated with an increased likelihood of P. aeruginosa59 infection and adrenal insufficiency in 48%.60
Nsaids: there is not enough information to support their role.61,62
Statins: they have anti-inflammatory properties, but there is not enough information either.63
Non-pharmacological treatment
Avoid lung irritants: avoid lung irritants: exposure to respiratory irritants should be avoided as far as possible, e.g. smoking and vaping, cleaning agents, dusts, fumes, gases, etc.
Systemic hydration: maintaining hydration is important to help decrease thick secretions.
Pulmonary rehabilitation (PR): undoubtedly brings benefit.64 Indicated for patients with diminished exercise capacity.65 Aerobic exercise (cycloergometer, elliptical) is recommended in stable patients with dyspnea mMRC = 2-4.45 To deepen into the subject, we suggest going to the pulmonary rehabilitation guidelines.
Airway clearance therapy: all patients should undergo physiotherapy regularly to remove airway secretions (Table 4).66
Bronchial hygiene improves cough67 by adequately expelling secretions and mucous plugs from the airway with manual techniques or devices (Table 7).68 The choice of a technique or device should be based on the amount and characteristics of the secretions, patient comfort, cost, and ability to use the device.69 The drainage of secretions is contraindicated in unstable situations.
Oscillatory positive expiratory pressure (PEP) devices combine with high-frequency oscillations to release secretions and move them into the mouth. Schemes of 6-10 deep inhalation cycles, 2-3 second breath hold, exhalation through the device creating oscillations and coughing. Oscillatory PEP improves quality of life, but not the amount of sputum, dyspnea, or lung function.70 Information about PEP during exacerbations or long-term use is missing.
Nutritional support. The protein-rich nutritional supplement enriched with hydroxy-beta-methylbutyrate (anti-catabolic and anti-inflammatory effect) showed a greater improvement in strength and physical functioning.71
Other medical therapies
Anti-GER: Suppression of gastric acid by use of H2 blocker or proton pump inhibitor is indicated in persistent and unexplained symptomatic patients or in those with ≥ 2 exacerbations/year. Anti-reflux measures must also be carried out.
Vaccines: review missing immunizations and promote the application of influenza vaccine (live attenuated viruses except in immunodeficiency). Pneumococcal polysaccharide vaccine or conjugate must be present.72 Both vaccines significantly reduce the number of acute infectious exacerbations during the first year compared to the influenza vaccine alone.73
Lung surgery and transplantation
Surgery (lobectomy or segmentectomy) is indicated in case of localized bronchiectasis or severe hemoptysis that does not respond to conventional treatment. Lung transplantation is the only solution in patients with advanced disease, whose survival is estimated to be < 2 years, once all available treatments have been used without obtaining a response.
Follow-up: patients should be asked at each visit about dyspnea and exercise tolerance, color, consistency, and estimated amount of sputum, as this information is useful in determining if a patient has an exacerbation.
Exacerbations, emergency department visits or hospitalizations, or if they were given antibiotics, including dose and frequency, should be recorded. Must have supervision in pulmonary rehabilitation programs.
Conclusions
AFILIACIONES
1Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas. Mexico City, Mexico.Conflict of interests: the authors declare that they have no conflict of interests.
REFERENCES
Aliberti S, Goeminne PC, O'Donnell AE, Assamit T, Al-Jahdali H, Barker A, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med. 2022;10(3):298-306. doi: 10.1016/s2213-2600(21)00277-0.
Barker AF. Clinical manifestations and diagnosis of bronchiectasis in adults. In: UpToDate, King TE, editor. UpToDate, Dieffenbach P. [Accessed on October 2022]. Available in: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-bronchiectasis-in-adults?search=Clinical%20manifestations%20and%20diagnosis%20of%20bronchiectasis%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
Barker AF. Bronchiectasis in adults: treatment of acute exacerbations and advanced disease. In: UpToDate accesado. Section editor: Stoller JK, Deputy editor: Paul Dieffenbach P. 2023. Available in: https://www.uptodate.com/contents/bronchiectasis-in-adults-treatment-of-acute-and-recurrent-exacerbations?search=Bronchiectasis%20in%20adults:%20Treatment%20of%20acute%20exacerbations%20and%20advanced%20disease&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
Martínez-García MA, Máiz L, Olveira C, Girón RM, De la Rosa D, Blanco M, et al. Normativa sobre el tratamiento de las bronquiectasias en el adulto. Arch Bronconeumol. 2018;54(2):88-98. Available in: https://www.archbronconeumol.org/en-spanish-guidelines-on-treatment-bronchiectasis-articulo-S1579212917303841
Olveira G, Olveira C, Doña E, Palenque FJ, Porras N, Dorado A, et al. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (Prospective, Randomised Study). Clin Nutr. 2016;35(5):1015-1022. doi: 10.1016/j.clnu.2015.10.001.
|
Table 1: Diagnostic approach: characteristic causes and tests.22-24 |
||
|
Category |
Specific examples/traits |
Diagnostic test |
|
Acquired bronchial obstruction (several produce localized bronchiectasis) |
||
|
Foreign body suction |
Peanuts, bone, tooth, etc. |
X-ray, CT scan; FBC |
|
Tumors |
Laryngeal papillomatosis; adenoma, endobronchial teratomas |
X-ray, CT scan; FBC |
|
Adenopathy |
Tuberculosis; histoplasmosis; sarcoidosis |
PPD; X-ray, CT scan; FBC |
|
COPD |
Chronic bronchitis |
PFT, symptoms |
|
Connectivopathies |
Polychondritis, amyloidosis |
Cartilage biopsy |
|
Mucoid impaction |
ABPA; bronchocentric granulomatosis; post-surgical |
Total and specific IgE aspergillosis; skin reaction, X-ray, CT scan; bronchial biopsy |
|
Congenital anatomical defects causing bronchial obstruction |
||
|
Tracheo-bronchial |
Bronchomalacia; bronchial cyst; cartilaginous deficiency (Sx Williams-Campbell); tracheobroncomegaly (Sx Mounier-Kuhn); ectopic bronchus; tracheoesophageal fistula |
X-ray, CT scan |
|
Vascular |
Intralobar sequestration, pulmonary arterial aneurysm |
X-ray, CT scan |
|
Lymphatics |
yellow nail syndrome |
History of dystrophy, slow-growing nails |
|
Immunodeficiencies |
||
|
IgG |
Congenital (Bruton type), agammaglobulinemia; selective deficiency (IgG2, IgG4); acquired Ig deficiency; variable common hypogammaglobulinemia; Nezelof Syndrome; «Naked lymphocyte syndrome» |
Quantitative Ig and subclasses; damaged response to pneumococcal vaccine |
|
IgA |
Selective deficiency with or without ataxia-telangiectasia syndrome |
Quantitative Ig |
|
Leukocyte dysfunction |
Chronic granulomatous disease (NADPH oxidase dysfunction) |
Dihydrorhodamine 123; oxidation test; tetrazolium nitroblue test, genetic testing |
|
Humoral immunodeficiencies (CXCR4 mutation, CD40 and ligand deficiency) |
WHIM syndrome; hypergammaglobulinemia M |
Neutrophil count; Ig levels |
|
Abnormal clearance of secretions |
||
|
Mucociliary defects |
Kartagener syndrome; ciliary dyskinesias |
X-ray, CT scan (situs inversus); bronchial biopsy; ciliary motility; electron microscopy of sperm or respiratory mucosa |
|
Cystic fibrosis |
Typical early infantile LH; late presentation with sinopulmonary symptoms |
Chlorine in sweat; genetic testing |
|
Young syndrome |
Obstructive azoospermia with sinopulmonary infections |
Spermatocrit |
|
Miscellaneous disorders |
||
|
Alpha-1 antitrypsina deficiency |
Absence or synthesis/abnormal function |
Alpha-1 antitrypsin levels |
|
Recurrent bronchoaspiration pneumonia |
Alcoholism; neurological disorders; lipoid pneumonia |
Medical record; X-ray, CT scan |
|
Connectivopathies |
Sjogren syndrome and Rheumatoid Arthritis |
Rheumatoid factor; antiSSA/antiSSB; salivary gland biopsy |
|
Toxic inhalation of fumes and dusts |
Ammonium; nitrogen dioxide, irritant gases; fumes; talc; silicates |
Medical record; X-ray, CT scan |
|
Post-transplant rejection |
Bone marrow, bronchiolitis obliterans (lung transplant) |
PFT; X-ray, CT scan |
|
Childhood infections |
Pertussis; measles |
Medical record |
|
Bacterial infections |
Staphylococcus aureus, Klebsiella, Pseudomonas aeruginosa |
Medical history; cultures |
|
Viral infections |
Adenovirus (types 7 and 21), influenza, herpes simplex |
Medical history, evidence of infection |
|
Other infections |
Histoplasmosis; Mycobacterium tuberculosis, non-tuberculous mycobacterium; mycoplasma |
Cultures; stains |
|
X-ray = simple chest X-ray. CT scan=computed tomography of the chest. FBC = fibrobronchoscopy. COPD = chronic obstructive pulmonary disease. ABPA = allergic broncho pulmonary aspergillosis. Ig = immunoglobulin. PFT = pulmonary function tests. Sx =syndrome. NADPH = nicotinamide adenine dinucleotide phosphate. Whim = warts, hypogammaglobulinemia, infections and myelocatexis. PPD = purified protein derivative. antiSSA =antibody Sjögren’ssyndrome A/Ro. antiSSB = antibody Sjögren’s syndrome B/La. |
||
|
Table 2: Bronchiectasis Severity Index (BSI).32,34 |
|
|
|
Score |
|
Age (years) < 50 50-69 70-79 > 80 |
0 2 4 6 |
|
Body mass index < 18.5 18.5-25 26-29 ≥ 30 |
2 0 0 0 |
|
FEV1 (% of predicted value) > 80 50-80 30-49 < 30 |
0 1 2 3 |
|
Medical research council dyspnea scale 1-3 4 5 |
0 2 3 |
|
Colonization by Pseudomonas aeruginosa No Yes |
0 3 |
|
Colonization by other organisms No Yes |
0 1 |
|
Radiological severity > 3 lobes or cystic bronchiectasis No Yes |
0 1 |
|
Hospitalization in the last year No Yes |
0 5 |
|
Exacerbations in the last year 0 1-2 ≥ 3 |
0 0 2 |
|
Score: mild 0-4 points, moderate 5-8 points, severe ≥ 9 points. |
|
|
Table 3: FACED Score.33,34 |
||
|
Variable |
Values |
Scores |
|
Exacerbations with hospital admission (previous year) |
No |
0 |
|
At least 1 |
2 |
|
|
FEV1 (% of predicted) |
At least 50% |
0 |
|
Less than 50% |
2 |
|
|
Age (years) |
Under 70 years of age |
0 |
|
At least 70 years of age |
2 |
|
|
Chronic bronchial infection (colonization) by Pseudomonas aeruginosa |
No |
0 |
|
Yes |
1 |
|
|
Radiological extension (number of lobes) |
1-2 |
0 |
|
More than 2 |
1 |
|
|
Dyspnoea (modified mMRC scale) |
0-II |
0 |
|
III-IV |
1 |
|
|
FACED = FEV1 Age, Chronic colonization, Extension, Dyspnea. FEV1 = expiratory volume in the first second. mMRC = modified Medical Research Council. Score: mild 0-2 points, moderate 3-4 points, severe 5-7 points. |
||
|
Table 4: Treatment of mild exacerbation. |
|||
|
Agent |
Selection |
Alternative |
Duration (days) |
|
Haemophilus influenzae |
Amoxicillin/clavulanate 875/125 mg every 8 h |
Amoxicillin 1-2 g every 8 h Ciprofloxacin 750 mg every 8 h Azithromycin 500 mg every 24 hours |
10-21 Azithromycin 3-5 |
|
Staphylococcus aureus |
Amoxicillin/clavulanate 875/125 mg every 8 h |
Amoxicillin/clavulanate 875/125 mg every 8 h |
10-21 |
|
MRS |
Linezolid 600 mg every 12 h PO |
Clindamycin 300-450 mg every 8 h |
10-21 |
|
Pseudomonas |
Ciprofloxacin 750 mg every 12 h PO |
Levofloxacin 750 mg every 24 h PO |
14-21 |
|
MRS = Methicillin-resistant Staphylococcus aureus. PO = PER OS (orally). |
|||
|
Table 5: Treatment of severe exacerbation. |
|||
|
Agent |
Selection |
Alternative |
Duration (days) |
|
Haemophilus influenzae |
Ceftriaxone 2 g/24 h IV |
Amoxicillin/clavulanate 500/125 mg 2 tab every 8 h PO |
14-21 |
|
Staphylococcus aureus |
Vancomycin 15-20 mg/kg/8 - 12 h IV |
Vancomycin 15-20 mg/kg/8-12 h IV |
14-21 |
|
MRS |
Linezolid 600 mg/12 h IV |
Vancomycin 15-20 mg/kg/8-12 h IV Ceftriaxone 600 mg every 12 h IV |
14-21 |
|
Pseudomonas |
Ceftazidime 2 g every 8 h IV + tobramycin 5-10 mg/kg every 24 h IV |
Imipenem 1 g every 8 h or piperacillin/tazobactam 4-8 g every 24 h or cefepime 2 g every 8 h or meropenem 2 g every 8 h or ciprofloxacin 400 mg every 12 h + amikacin 15-20 mg/kg every 24 h or gentamicin 5-7 mg/kg every 24 h |
14-21 |
|
MRS = Methicillin-resistant Staphylococcus aureus. PO = PER OS (orally). IV = intravenous. |
|||
|
Table 6: Treatment of chronic infection. |
||
|
Agent |
Nebulized |
Oral or intravenous |
|
Pseudomonas |
Tobramycin (solution for nebulization): 300 mg/5 mL twice daily 28 days of treatment followed by 28 days of rest in e-Flow®, Pari LC plus® Gentamicin (inhaled intravenous formulation): 80 mg twice daily continuous treatment |
If despite nebulized treatment poor clinical control persists, associate an oral or intravenous antibiotic with activity according to antibiogram, on demand or in cycles |
|
MRS |
Vancomycin (intravenous formulation administered by nebulized route): 250 mg/2 times a day continuous treatment |
If the response is insufficient or there is intolerance, add or replace vancomycin with IV linezolid |
|
Other germs |
Gentamicin: 80 mg/2 times daily or any of those used for pseudomone continuous treatment |
If the response is insufficient or there is intolerance, consider adding (or replacing) the nebulized antibiotic with an oral one according to sensitivity |
|
MRS = Methicillin-resistant Staphylococcus aureus. |
||
|
Table 7: Airway cleaning /bronchial hygiene. |
||
|
Technique |
Advantages |
Comment / disadvantage |
|
Directed cough |
Cheap, simple |
Chest pain may limit |
|
Regular exercise |
Economical, strengthens respiratory and peripheral muscles |
|
|
Autogenous breathing |
Control your breathing |
Requires patient cooperation |
|
Forced expiration |
Helps control breathing |
Requires patient learning |
|
Thoracic physiotherapy (CPT; postural drainage, mechanical or manual thoracic percussions) |
Most tested in cystic fibrosis |
Needs assistant, difficult to place, hypoxemia, sometimes worsens gastroesophageal reflux |
|
Positive expiratory pressure (PEP) |
Easy, cheap |
Device needs cleaning |
|
Oscillatory PEP (e.g., acapella device with flutter valve) |
Easy, economical, adds vibration to the airways |
Device needs cleaning |
|
High frequency chest wall oscillation vest with inflatable bladder |
Extensive experience |
Pain may limit; needs power outlet |
|
High frequency chest wall oscillation vest with mechanical oscillators |
Doesn’t hurt compared to inflatable bladder |
May be mobile; small batteries in vest; closest to CPT |
|
CPT = chest physical therapy. |
||


