Role of the antimicrobial bactericidal permeability increasing protein in respiratory diseases
Guzmán-Beltrán, Silvia1; Luna-Villagómez, Heidi A1,2; Páez-Cisneros, César A1,2; Santos-Méndez, Mayra A1,3; Reyes-Lazcano, Marcos E1,4
Guzmán-Beltrán, Silvia1; Luna-Villagómez, Heidi A1,2; Páez-Cisneros, César A1,2; Santos-Méndez, Mayra A1,3; Reyes-Lazcano, Marcos E1,4
ABSTRACT
The bactericidal permeability increasing protein is a molecule of the immune system which participates in the defense against pathogens. This protein binds to Gram-negative bacterial membranes altering permeability and inducing lysis. It also binds to free lipopolysaccharides, inhibiting Theo sígnala chat leads no inflamarían. In admitían, tris porten induces he opsonization activity contributing to phagocytosis. The role of the bactericidal permeability increasing protein, during infections by Gram-positive bacteria is still controversial. However, this protein increases in meningitis caused by Streptococcus pneumoniae and Neisseria meningitidis and during respiratory infections caused by the influenza A virus, possibly modulating the production of proinflammatory cytokines. In other respiratory disorders such as chronic obstructive pulmonary disease, cystic fibrosis, and asthma, the production of autoantibodies against this protein is recurrent. This antibody production reduces the bactericidal permeability increasing protein serum levels, decreasing antimicrobial defense against pulmonary infections. Therefore, it is essential to understand the role of this protein during respiratory diseases to propose possible therapies to improve patient health.KEYWORDS
bactericidal protein that increases bacterial permeability, microbial infections, inflammation and autoimmunity.Abbreviations:
Introduction
The purpose of the immune system is to protect organisms from infectious agents.1,2 Macrophages and neutrophils are cells of the immune system with the ability to ingest pathogens, effectively destroying them in phagosomes by generating reactive oxygen and nitrogen species (ROS and RNO) and protein-based antimicrobial molecules, contributing to a multifaceted, coordinated and highly effective defense. ROS produced by neutrophils and macrophages are: superoxide anion (O2-), hydrogen peroxide (H2O2) and hypochlorous acid (HOCl); while RNS are: nitric oxide (NO), nitrogen dioxide (NO2) and peroxynitrite (ONOO-).1 These species are highly toxic to pathogens, leading to their death.
However, some pathogens, such as Candida albicans and Staphylococcus aureus, are resistant to oxidative attack; therefore, the production of antimicrobial peptides and proteins is necessary to eliminate the pathogen.3,4 Antimicrobial peptides include defensins, granzymes, cathelicidins, and other cationic peptides and proteins such as lactoferrin and bactericidal/permeability increasing protein (BPI) to ensure the elimination of pathogens.2,5
The aim of this review is to show the importance of BPI during various respiratory diseases. The mechanisms by which BPI contributes to pathogen clearance will be explored and a comprehensive view of the importance of BPI as an essential component of the immune response will be provided.
General characteristics of BPI
The BPI protein is a member of the family of lipid transporter proteins, which can bind to various lipid molecules such as lipopolysaccharides (LPS). The different members of the family include proteins involved in the innate immune response, such as: LPB (Lipopolysaccharide-Binding Protein), PLUNC (Palate, Lung, and Nasal epithelium Clone), CETP (Cholesteryl Ester Transfer Protein) and PLTP (Phospholipid Transfer Protein).6
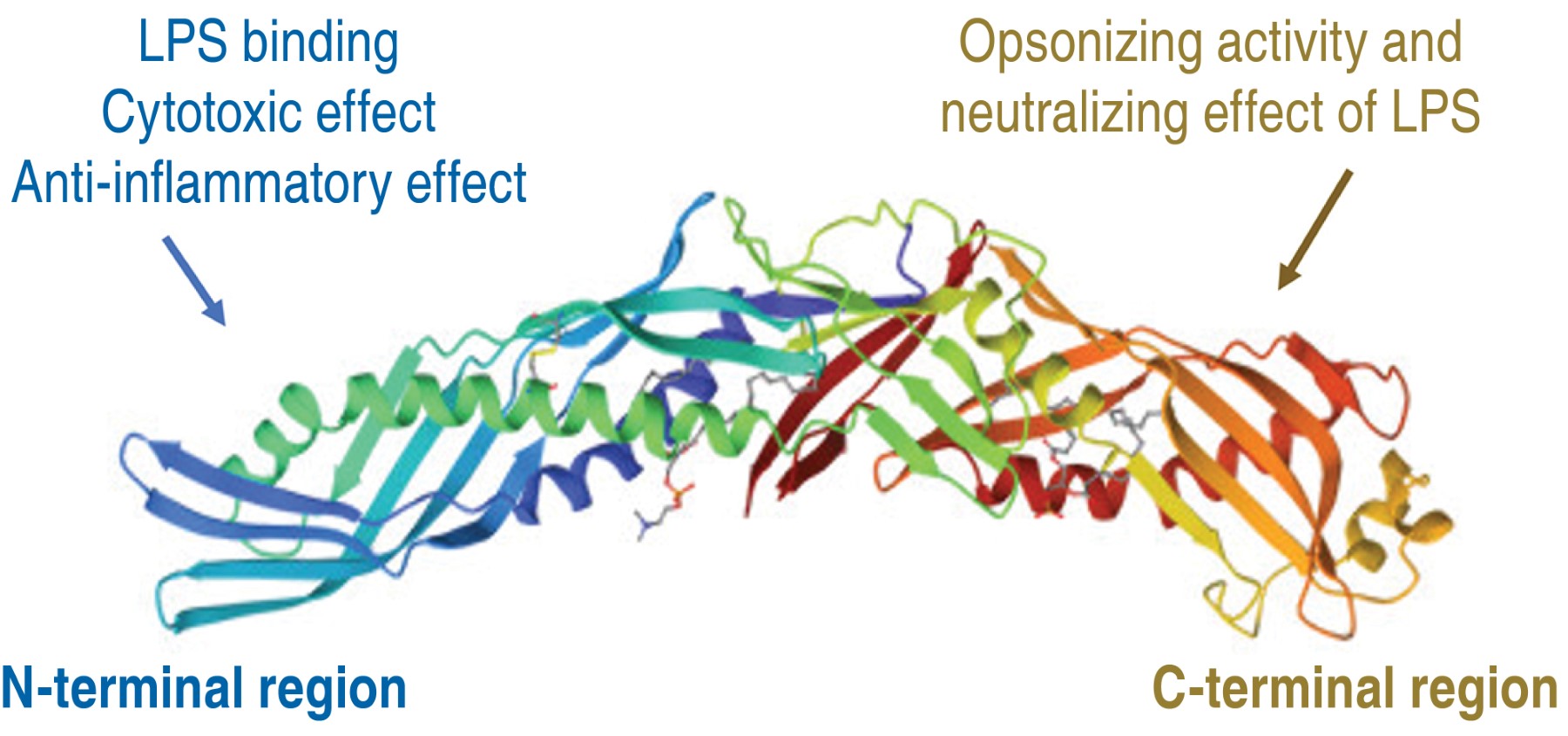
BPI is expressed in various cell types such as: neutrophils, polymorphonuclear cells, eosinophils, macrophages, platelets and epithelial cells.7 BPI is composed by 456 amino acid residues, with a molecular weight of 55,000 Daltons (Da), of cationic nature.8 In X-ray crystallography analysis, BPI has a bipartite boomerang-shaped structure composed of two main domains: an amino-terminal domain (N-terminal) and a carboxy-terminal domain (C-terminal), separated by a proline-rich region. It has two apolar lipid binding sites, one in each half of the molecule, allowing it to bind to the hydrophobic acyl chains of LPS.9
The primary structure of BPI reveals that the N-terminal region is positively charged due to the abundance of basic residues, mainly lysine. The cationic characteristic of antimicrobial proteins and peptides allows them to interact with LPS found in the negatively charged bacterial envelope.10 BPI is an essential molecule in the immune response, as it has antimicrobial action, opsonizing effect and anti-inflammatory activity.7 The three-dimensional structure of the protein is illustrated in Figure 1.
Importance of BPI in respiratory diseases
Respiratory diseases represent a major global problem. Infectious diseases cause millions of deaths worldwide, for example, influenza A virus infections occur worldwide and cause between 3 and 5 million severe cases and between 0.29 and 0.65 million deaths. Each year, tuberculosis kills 1.6 million people, while 2.5 million die of pneumonia, 30% of whom are children under five years of age. Other non-infectious respiratory diseases that cause human losses are: chronic obstructive pulmonary disease (COPD), which is the third leading cause of death worldwide, causing 3.23 million deaths in 2019 alone. Asthma causes an average of 1,000 deaths per day;11 as for cystic fibrosis (CF), despite being a rare disease, an average of 300 people are born with this condition in Mexico.12
In several respiratory diseases, it has been described that the BPI protein presents high levels, playing an essential role in several diseases. This can be explained because it has the capacity to fight infections, regulate inflammation and modulate the immune response in the lungs, which makes it a key factor in the protection of the respiratory system. It is possible that BPI has potential as a diagnostic marker or even has therapeutic use in treating infections. In fact, BPI has been shown to play a crucial role in the prevention and treatment of respiratory diseases such as viral and bacterial infections, as well as COPD, CF and asthma.
BPI in bacterial infections
The ability of BPI to bind to LPS allows it to have antibacterial action towards Gram-negatives such as Escherichia coli, Salmonella typhimurium, Shigella and Enterobacter spp, being effective at nanomolar concentrations.2,10
The selective binding of BPI to lipid A of LPS and to bacterial surface phospholipids of living bacteria causes increased membrane permeability and formation of pores in the wall, allowing entry of other antimicrobial molecules and generating loss of proton motive force, lysing the bacteria. The positive charge of the amino-terminal domain of BPI interacts with the negative charge of LPS.13
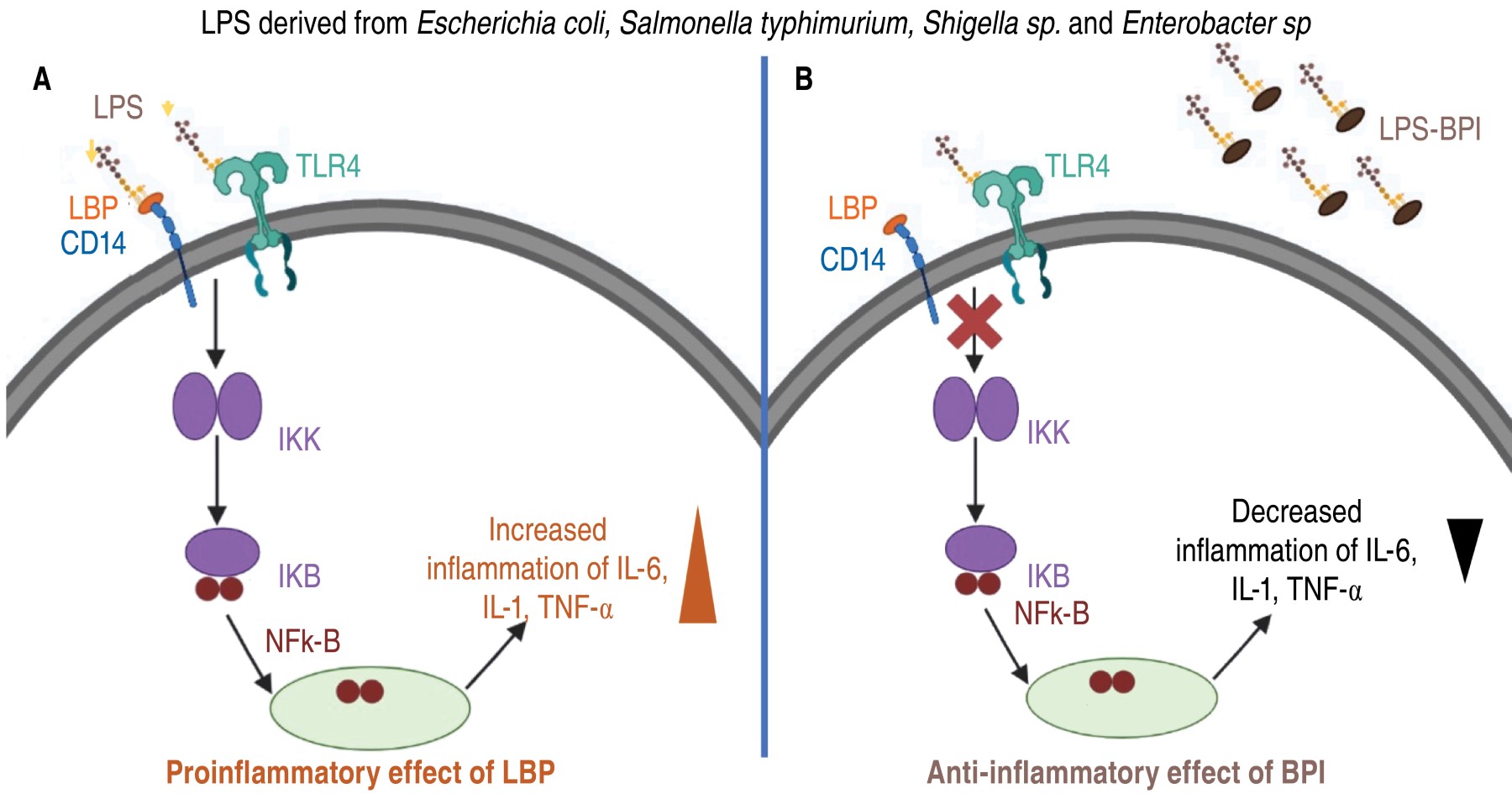
BPI has an anti-inflammatory effect by preventing the production of proinflammatory cytokines triggered by LPS. BPI belongs to the above-mentioned family of lipid transfer proteins, which also includes lipopolysaccharide-binding protein (LBP), which is present in serum.14,15 Unlike LBP, which facilitates proinflammatory activation of monocytes by LPS, binding of BPI to LPS reduces its ability to trigger endotoxin activation.16 LBP catalyzes and disperses LPS aggregates and binds LPS monomers to CD14/TLR-4 receptor complexes, triggering the release of proinflammatory cytokines.16 Due to its high affinity for LPS, BPI increases the size of LPS aggregates, which prevents LPS from interacting with LBP and reduces the production of proinflammatory cytokines by macrophages.16-19 Figure 2 illustrates the anti-inflammatory mode of action of BPI and the opposite effect of LBP protein.
Likewise, the opsonizing effect of BPI has been described, which is attributed to the carboxyl domain of the protein, since it enhances the delivery of vesicles derived from the outer membrane of Gram-negative bacteria to dendritic cells. Preincubation of E. coli with 50 nM BPI was shown to increase phagocytosis in neutrophils in the absence of serum, but increases to 100% in the presence of serum, which accelerates opsonization by complement proteins.20
In a model of lung infection caused by Pseudomonas aeruginosa, Streptococcus pneumoniae or Streptococcus agalactiae, it was shown that a recombinant BPI21 fraction (21 amino acids of the N-terminal region) improved the survival of mice infected with these strains by inducing apoptosis of infected cells. In addition, bronchial lavage cells from infected mice were shown to exhibit high expression of BPI.21
On the other hand, in patients with meningitis caused by S. pneumoniae and Neisseria meningitidis, there is significant increase of BPI in cerebrospinal fluid.22 Furthermore, they demonstrated that BPI can bind specifically to teichoic acids and lipopeptides, suggesting that BPI is a molecular pattern recognition molecule associated with bacteria in general. Furthermore, interaction of BPI with molecules derived from Gram-positive bacteria induces the expression of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6).22
It was also shown in a model of human macrophage infection with Mycobacterium tuberculosis that treatment with inflammation-resolving lipids such as maresin 1 and resolvin D induced BPI expression and significantly decreased intracellular growth of mycobacteria, suggesting an important role in infections caused by Gram-positive bacteria.23
BPI in viral infections
Influenza is a common infectious disease, the most frequent causative agent being the influenza A virus, which is a very successful pathogen, circulating constantly in diverse hosts such as humans, pigs, horses, dogs and birds.24 Annual epidemics of seasonal influenza result in millions of infected people worldwide.25
In an in vitro model, influenza A virus variants (H1N1, H3N2 and H5N1) were found to induce the release of BPI from human granulocytes.26 It was found that a recombinant BPI27 fraction (27 amino acids of the N-terminal region) is able to inhibit viral infectivity and modify the structure of the influenza A H1N1 virus in human mononuclear cells.
In the same model, they also showed that cells infected with influenza A H1N1 virus treated with the BPI27 fraction significantly decreased interferon alpha (IFN-α) and IL-6 production in a concentration-dependent manner.26
Although there is high basal expression of BPI in diverse cell types such as gastrointestinal epithelial cells, dermal fibroblasts, basal epithelial cells of the ducts of the excretory lacrimal glands and of the genital tract in humans, the role of BPI in various infections and its contribution to the elimination of viral pathogens is unknown.27-30
BPI in patients with chronic obstructive pulmonary disease (COPD).
COPD is characterized by persistent respiratory symptoms such as dyspnea, chronic cough and mucoid sputum production, decreased airflow due to airway or alveolar abnormalities. As for its pathophysiology, there is an increase in the number of goblet cells, hyperplasia of the mucous glands, collapse of the airways due to destruction of the alveolar wall, narrowing and decrease in the number of small airways, fibrosis of the lung parenchyma and recurrent infections.31
The main pathogen associated with COPD is P. aeruginosa, which contributes to the production of autoantibodies against BPI (anti-BPI), decreasing the ability to clear the bacteria and inducing an exacerbated inflammatory response.32 It was shown that anti-BPIs are formed from a pathway dependent on CD18, a β-2 integrin, necessary for phagocytosis that acts if BPI and microbial antigen are taken up simultaneously and together are presented to major histocompatibility complex (MHC) class II molecules on the cell surface to generate the production of autoreactive antibodies against BPI.33 Anti-BPI production in COPD patients causes increased neutrophil recruitment and increased production of inflammatory cytokines such as TNF-α, interleukin-1 beta (IL-1β) and IL-6, decreasing the antimicrobial and anti-inflammatory effect. In addition, patients who produce anti-BPI present pulmonary dysfunction, manifesting a decrease in the total exhaled fraction in the first second (FEV1) and total exhaled volume fraction (FVC).32
In another study, a group of volunteers (all men) with COPD exhibited decreased plasma BPI concentration compared to the control group (10.6 ± 2.2 versus 23.4 ± 2.1 ng/mL, p < 0.0001). Indeed, there was an association of low BPI levels with patient severity. Lower BPI leads to increased circulating LPS by increasing TNF-α levels, which generates greater inflammation and, therefore, greater clinical manifestations in COPD patients.34
BPI in patients with cystic fibrosis (CF)
During CF or mucoviscidosis, thick, viscous mucus is produced, causing obstruction of the ducts of the organs where it is located, with the pancreas and lungs being the most compromised organs. The accumulation of thick mucus leads to blockage in the airways, as well as repeated episodes of inflammation, lung damage and recurrent respiratory infections that affect the quality of life of thousands of people worldwide.35
CF is caused by mutations in the gene that produces the CF transmembrane conductance regulator (CFTR) protein. This protein is responsible for regulating the flow of salt and fluids in and out of cells in different parts of the body. In CF, the CFTR protein may be absent or dysfunctional, resulting in decreased water movement, which causes more thick mucus to accumulate.36
CF patients have recurrent infections with S. aureus, Streptococcus pneumoniae, Burkholderia cepacia, Haemophilus influenzae, Achromobacter xylosoxidans, Stenotrophomonas maltophilia, P. aeruginosa and some mycobacteria.37 Seventy percent of patients may be co-infected by different pathogens. For example, S. aureus and P. aeruginosa can persist together with H. influenzae or S. pneumoniae; however, the microorganism that most frequently colonizes the respiratory tract in patients with CF is P. aeruginosa, which generates chronic pulmonary deterioration.38
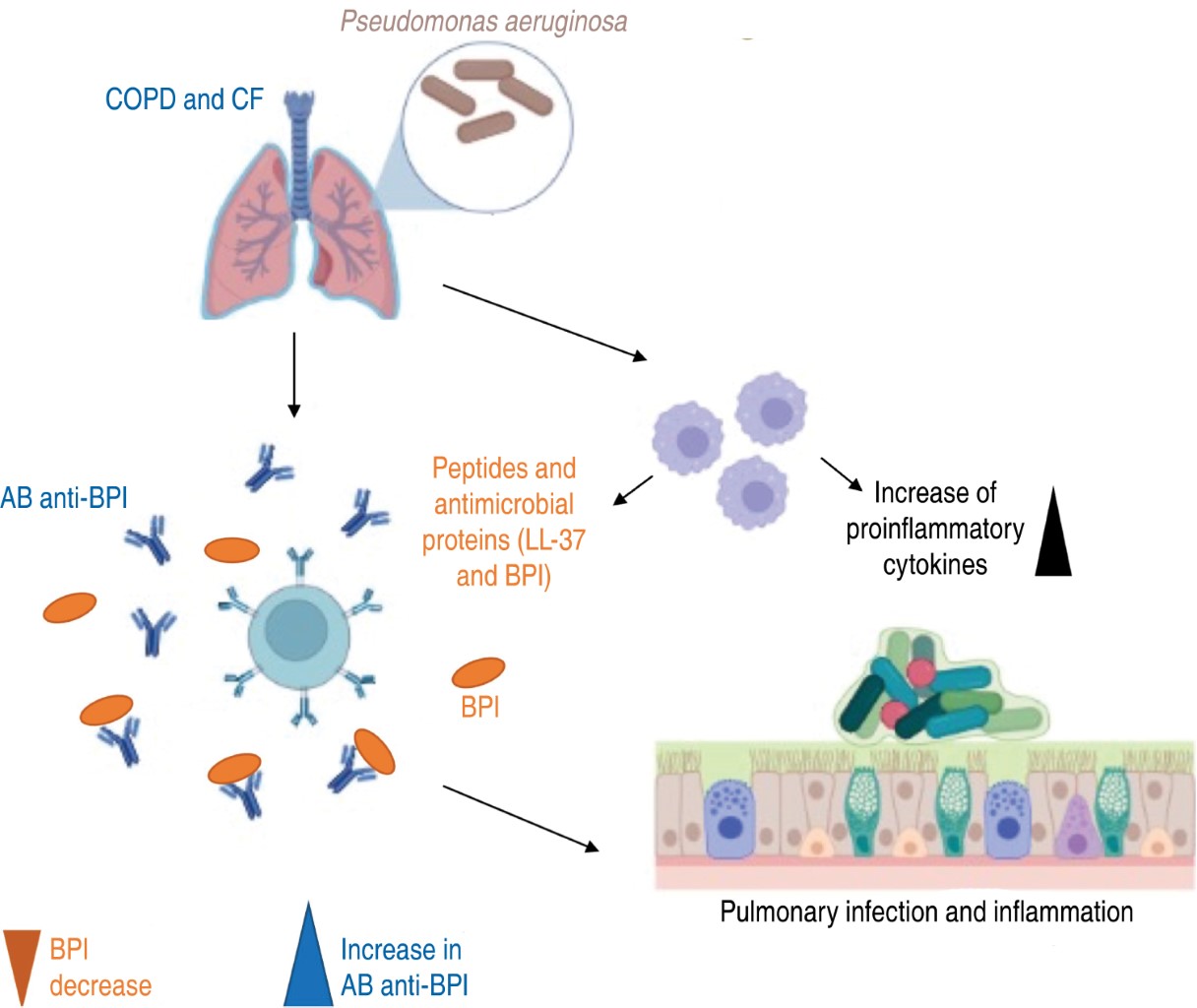
It has been shown that in CF patients during infection with P. aeruginosa, antibodies to the bacterium and to the BPI protein are also induced.35 These anti-BPI autoantibodies have a variable prevalence in at least 50% of people with CF. Therefore, anti-BPIs are considered a useful prognostic marker, as patients with high serum levels have increased lung damage leading to lung transplantation or premature death.39,40 Figure 3 illustrates how autoantibodies are generated and their involvement during an infection and their impact on the overall inflammatory state.
BPI in patients with asthma
Asthma is a heterogeneous and multifactorial disorder, characterized by reversible symptoms and it appears in episodes. However, as the disease progresses, permanent airway changes appear, such as smooth muscle hypertrophy and hyperplasia, as well as hypersecretion of the mucous glands.41,42
It has been shown that people with asthma have high levels of BPI. The serum BPI concentration of patients with uncontrolled asthma was three times higher (18.10 ± 13.48 ng/mL) compared to healthy controls (6 ± 2.27 ng/mL). Even patients with controlled asthma showed increased serum BPI (12.83 ± 6.04 ng/mL) compared to control. Then, when comparing serum BPI concentration with clinical indicators of patients with asthma, no significant relationship was found between BPI, eosinophil levels, IgE antibodies, fractional exhaled nitric oxide (FeNO) and percentage of FEV1. However, there is a significant positive correlation between BPI concentration and C-reactive protein, suggesting BPI as a possible biomarker for asthma.43
Conclusion
It has been demonstrated that BPI has a direct antimicrobial effect, neutralizes endotoxins and prevents the generation of antibiotic resistant strains due to its protein nature. In addition, BPI has no toxic effects, induces opsonization and has an anti-inflammatory effect; therefore, it is possible that BPI generates synergy with conventional antibiotics and antivirals. BPI has proven to be a promising therapeutic molecule that can control infection and inflammation in different diseases. A viable alternative could be to use recombinant BPI and induce the expression of endogenous BPI in the host, in order to contribute to the elimination of pathogens even in diseases where anti-BPI antibodies are generated.
AFILIACIONES
1Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City, Mexico 2Universidad Nacional Autónoma de México 3Universidad Autónoma Metropolitana 4Benemérita Universidad Autónoma de Puebla.Conflict of interests: the authors declare that they have no conflict of interests.
REFERENCES
Gazzano-Santoro H, Parent JB, Grinna L, Horwitz A, Parsons T, Theofan G, et al. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60(11):4754-4761. doi: 10.1128/iai.60.11.4754-4761.1992.
Kirschning CJ, Au-Young J, Lamping N, Reuter D, Pfeil D, Seilhamer JJ, et al. Similar organization of the lipopolysaccharide-binding protein (LBP) and phospholipid transfer protein (PLTP) genes suggests a common gene family of lipid-binding proteins. Genomics. 1997;46(3):416-425. doi: 10.1006/geno.1997.5030.