Pancreaticopleural fistula, a rare complication of pancreatic pseudocyst. Presentation of two cases
Hernández-Padilla, Sergio Luis1,2; Inocencio-Ocampo, Aleksander Eduardo2; Vázquez-Minero, Juan Carlos1
Hernández-Padilla, Sergio Luis1,2; Inocencio-Ocampo, Aleksander Eduardo2; Vázquez-Minero, Juan Carlos1
ABSTRACT
The presence of pancreaticopleural fistula is a rare complication of acute or chronic pancreatitis, and it can also occur in cases of pancreatic trauma. We report two cases of pancreatic pseudocyst that communicates towards the left hemithorax, both males aged 39 and 27 who, as a history, refer to intense chronic alcoholism. They present with long-term chest pain and dyspnea; pleural effusion is documented in imaging studies. When the endopleural tube is placed, the effusion appears dark and persists with a high output; when carrying out a study of the pleural fluid chemistry, a considerable elevation of pancreatic enzymes is identified, for which reason they are operated on by left posterolateral thoracotomy. A transdiaphragmatic fistula was identified in the first case and a transhiatal fistula in the second. In both cases, drainage plus lavage and decortication with closure of fistulas and placement of pleural drainage were performed. Both patients presented favorable evolution without requiring another procedure.KEYWORDS
pancreatic pseudocyst, pancreatic pleural fistula, pancreatitis, pleural effusion.Introduction
The presence of pancreaticopleural fistula (PPF) is a rare complication of chronic, acute pancreatitis or pancreatic trauma. The contents of a pancreaticopleural fistula can enter the thoracic cavity through the esophageal hiatus, the costodiaphragmatic angle and even through the diaphragm.1-3 Pleural effusion may occur in patients with acute pancreatitis in up to 9%; other pulmonary complications of acute or chronic pancreatitis are pneumonitis, atelectasis, dyspnea with respiratory distress.4-6 Cases of PPF have been described in only 0.4% of patients with pancreatitis and in up to 4.5% of those with pancreatic pseudocyst, especially in those associated with alcoholism.1,4,6 The most commonly described symptoms are dyspnea, chest pain and cough of chronic manifestation, as well as the presence of pleural effusion, which is more frequent on the left side.7-9 The mechanism occurs in the presence of a well-demarcated or ruptured pseudocyst, or a pancreatic duct where enzymatic activity smoothes the tissues, which favors a pleuroperitoneal communication that transforms into a fistula from the cyst into the pleural cavity.8-10 Extremely high pleural fluid amylase levels (> 50,000 IU/L) in addition to imaging studies in correlation with the patient's clinical presentation should suggest the presence of PPF.9,10 The endoscopic retrograde cholangiopancreatography approach has been described for the treatment of PPF, as well as the thoracic approach.10
We present two cases that were approached by thoracotomy with drainage, lavage and decortication, in addition to the use of postoperative endopleural probes, which achieved remission in both patients.
Presentation of cases
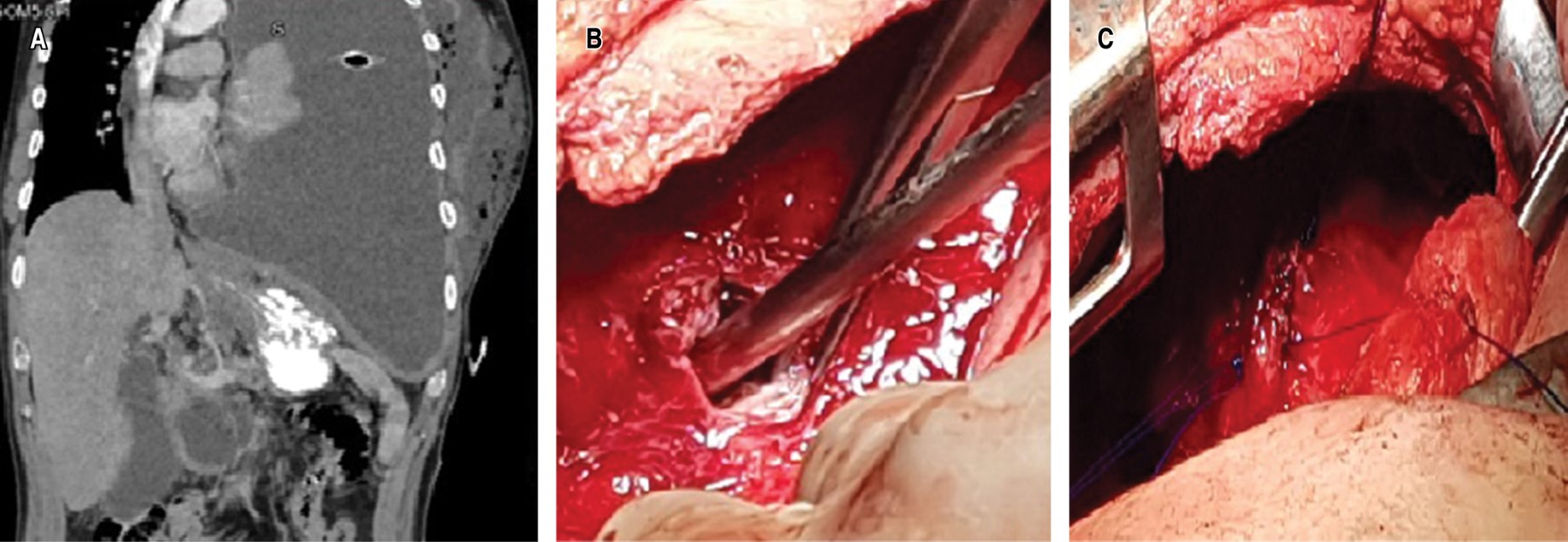
Clinical case 1. 39-year-old man, with a history of chronic alcoholism, with episodes of recurrent abdominal pain. His condition began three months prior to his admission, with pleuritic pain in the left hemithorax, progressive dyspnea and attack to the general condition; a chest X-ray was taken in which pleural effusion was identified. A thoracentesis was performed and a thick, dark pleural fluid was obtained, which, upon cytological and cytochemical analysis, showed amylase levels of 16,043 U/L and Light criteria for neutrophilic exudate. Subsequently, on performing computed axial tomography (CT) of the thorax and abdomen (Figure 1A), free fluid communication of the abdomen with the left hemithorax was identified, so it was decided to take the patient to the operating room to perform posterolateral thoracotomy; Parietal pleura was found to be 8 mm thick and visceral pleura 5 mm thick, abundant fibrin material and pus in the cavity, a fistulous tract between the abdominal cavity and left diaphragm of 1 cm in diameter (Figure 1B), with a collection of 100 mL of whitish liquid. Lavage and decortication were performed until adequate pulmonary expansion was achieved, the fistulous tract was debrided; edges were identified and the defect was closed with nonabsorbable 2/0 polypropylene suture (Figure 1C); pulmonary reexpansion was verified. After the treatment, the patient had an adequate clinical evolution; the endopleural drains, with serohematic output that decreased until their removal five days after the procedure. A control CT scan was performed, which showed a decrease in the initial pancreatic collection. After evaluation by the Gastroenterology Service, conservative management was left. At six months of surveillance with CT of the thorax, without evidence of complications.
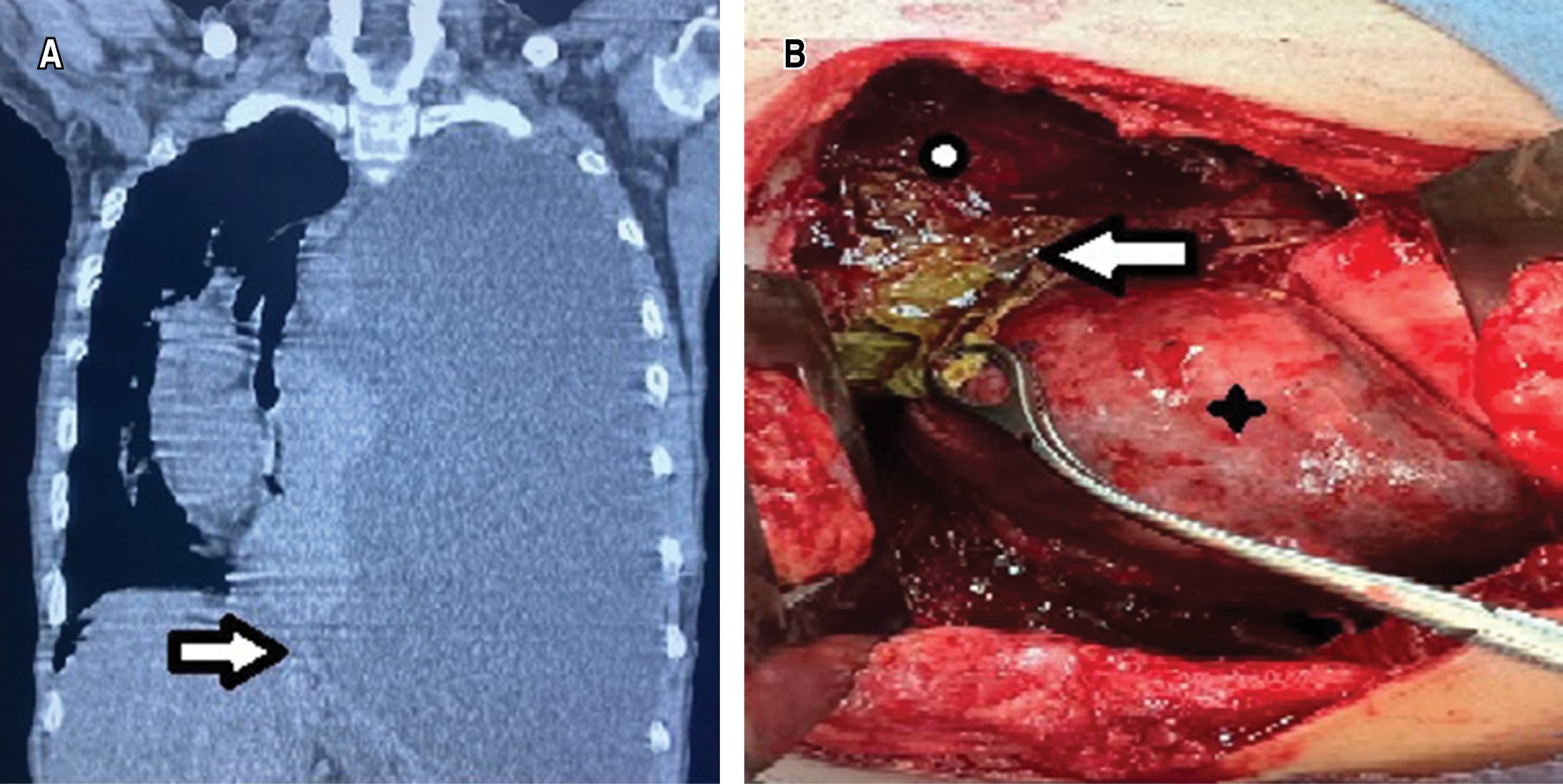
Clinical case 2. A 27-year-old man with a history of alcoholism since he was 14 years old and episodes of abdominal pain since one year prior to the assessment, for which he sought hospital care. He reported progressive dyspnea and increased abdominal pain, in addition to chest pain. Chest CT scan was performed and reported left hemithorax occupation associated with massive pleural effusion, with displacement to the right of mediastinal structures (Figure 2A). A left endopleural tube was placed, which, after placement, persists with an output of approximately 600 mL daily for two weeks, with a dark appearance and liquid consistency. He continued with respiratory deterioration, so he was referred to the Thoracic Surgery Area where pleural fluid analysis was requested, which revealed hematic exudate with elevated levels of amylase (195 U/L) and lipase (3,095 U/L). In addition, he showed leukocytosis and thrombocytosis in the blood biometry report. An abdominal CT scan was performed and a peripancreatic abdominal collection corresponding to pancreatic pseudocyst was identified. It was decided to go to the operating room; lavage and left decortication were performed; pleural effusion secondary to pancreatic pseudocyst communicated to the left hemithorax through the esophageal hiatus, pulmonary trapping, septated pleural effusion and left fibrothorax were identified (Figure 2B). Postoperatively, the pleural drains produced a low output, serohematic appearance (30-100 mL/day); they were removed on postoperative days five and seven. A control CT scan was performed where minimal abdominal collection persisted. After evaluation by the Gastroenterology Service, it was decided to maintain expectant management. The patient had a good postoperative evolution and was discharged on postoperative day 10. Post-surgical follow-up, at one month and three months with simple chest X-ray and at six months with chest CT, there was no evidence of complications.
Discussion
Pancreaticopleural fistula is a rare complication that occurs in patients with pancreatic pathology. Its pathophysiology describes a leaking pancreatic duct or a pseudocyst that can access the pleural cavity through a diaphragmatic hiatus or directly transdiaphragmatic; communication in the costodiaphragmatic angle has also been described.1
In our patients, the communication from the pseudocyst to the thorax was transdiaphragmatic and through the esophageal hiatus. There are no clear guidelines for the management of PPF because it is a rare entity; it has been reported that 50% of PPF will close with conservative measures, with subsequent resolution of the effusion.4,10
PPF is an infrequent entity and difficult to diagnose because it presents nonspecific clinical manifestations; this generates a delay in diagnosis that requires a high index of suspicion. Some differential diagnoses are: parapneumonic pleural effusion, as well as that associated with neoplasia; the history of alcoholism, young patients and associated abdominal pain, favor the diagnosis. The average time to diagnose PPF is about five weeks.4,7,10 In the cases we present, in addition to being cases of several weeks of evolution, the presence of persistent dark-colored effusion, the history of alcoholism, the pleural fluid study, as well as the imaging studies, both in the abdominal region and in the thorax, allowed us to reach the diagnosis, which coincides with the type of approach referred to in the literature for this type of patients.1,4,7,10
As mentioned, in most cases, treatment is conservative without requiring any surgical intervention to the thorax. There are options for the management of these patients as described in a series of four cases reported by Munirathinam M and collaborators in India, which were resolved by endoscopic management of the pancreatic pseudocyst, total parenteral nutrition therapy, octreotide and thoracic drainage with favorable results without requiring thoracic surgery.8-10
Surgical treatment is indicated in case of failure of medical treatment (3-4 weeks are considered) or obstruction of the pancreatic duct. Or, when there are loculated effusions that cannot be resolved by placement of thoracic drainage. Operative mortality is 3 to 5%. Recurrence has been reported in up to 11% of cases. The long-term outcome is good in 80-95% of cases, with an overall PPF mortality of 5%.1,7,9,10
In our cases, the lack of response to treatment and the long evolution of these cases led to the decision of surgical management. Washing, decortication and dismantling of the fistula were the basis for its successful resolution with a shorter evolution of two weeks in comparison with stays of three and up to six weeks after treatment.1,4,9,10
Conclusions
Pancreaticopleural fistula is a rare and difficult to diagnose complication, requiring a well performed study protocol combined with high suspicion of this entity, CT of the thorax and abdomen, as well as pleural fluid including pancreatic enzyme values.
Surgical management is a resource that should be considered in cases that do not respond to endoscopic management of the pseudocyst, or in patients with thoracic complications that require surgical approach.
Acknowledgments
To the sub directorate of Surgery and the head of the Cardiothoracic Surgery Service of the National Institute of Respiratory Diseases Ismael Cosío Villegas, Mexico City.
AFILIACIONES
1Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas. Mexico City, MexicoConflict of interests: the authors declare that they have no conflict of interests.
REFERENCES