Outpatient follow-up of patients with broncopulmonary dysplasia
Jamaica-Balderas, Lourdes María del Carmen1; Fonseca-Larios, Waldo Moisés1; Romero-Mena, Fabián1; Barragán-González, Arelis1
Jamaica-Balderas, Lourdes María del Carmen1; Fonseca-Larios, Waldo Moisés1; Romero-Mena, Fabián1; Barragán-González, Arelis1
ABSTRACT
KEYWORDS
Bronchopulmonary dysplasia, lung diseases, infant premature, premature birth, premature diseases.Introduction
Bronchopulmonary dysplasia (BPD) was described by Northway, Rosen and Porter (1967) as a lung disease in preterm infants requiring prolonged mechanical ventilation and high levels of supplemental oxygen1,2 and is defined by total duration of supplemental oxygen use, positive pressure requirement and gestational age, as well as oxygen dependence at 36 weeks postnatal age.3,4
The incidence in care centers varies between 20 and 75%.5 Cohort studies such as ELGAN, Canadian Neonatal Network, Korean Neonatal Network, Vermont-Oxford Network and Swiss Neonatal Network, and studies conducted in China, Taiwan and India, show prevalences between 11 and 50%, due to differences related to gestational age or birth weight criteria associated with the diagnosis.6
The variation in neonatal outcomes identified in multicenter and multinational cohorts may result from differences in coverage, population characteristics, structure of perinatal health care, case definitions, quality and processes of care in different countries.1
Risk factors include intrauterine growth restriction, male sex, chorioamnioitis, race, smoking,1 and even genetic risk.7-10
Since 2005, the prevalence of BPD from the Vermont Oxford Network has decreased from 31 to 28%. Globally, BPD rates ranged from 13 to 32% in the iNEO (International Network for Evaluation Outcomes in Neonates) between 2007-2010.6,11-15
Recent evaluations in the USA indicate that BPD develops in approximately 10% of preterm infants born between 28 and 31 weeks, and in 40% of preterm infants younger than 28 weeks.16 In Europe, 10 to 20% of preterm infants between 23 and 31 weeks developed BPD.16 In Mexico the prevalence of preterm infants is 10%, of which 8 to 12% are less than 1,200 g or less than 32 weeks, being this the population susceptible to develop BPD.17
Although BPD continues to be the most frequent complication in children under 30 weeks and low birth weight, in the last 50 years management has evolved through the use of prenatal corticosteroids, advanced techniques in neonatal care and the use of surfactant, allowing newborns with BPD to have better survival and a lower risk of mortality, although this favors an increase in prevalence.1,18,19
Despite all efforts to prevent lung injury, it remains the most prevalent chronic lung disease in the preterm infant,20 characterized by uniform inflammation, low-grade fibrosis, absence of airway epithelial metaplasia, smooth muscle hypertrophy, larger alveoli, and pulmonary vascular dysfunction.21
Care of the extremely premature infant requires hospitalization for approximately 60 days, and in some cases rehospitalization after discharge.5 During their first year of life, 49% require readmission.22,23 Follow-up studies are important because they allow visualization of pulmonary involvement, asthma-like symptoms, pulmonary hypertension and exercise intolerance with altered response to hypoxia.24
In this work, our aim was to retrospectively describe the demographic characteristics, associated factors and comorbidities in patients with BPD who attended pediatric Pneumology outpatient clinic between 2014 to 2018.
Material and methods
An observational, descriptive, retrospective study was conducted in 386 patients with a diagnosis of BPD who met the definition according to Bancalari (Table 1), and were seen in the outpatient Pneumology department of the Hospital Infantil de México "Federico Gómez" between 2014 and 2018. Patients with cyanosinging congenital heart disease or incomplete clinical history were not included.
Data for this study were taken from medical records and information on care received in the first two years of life was evaluated. Qualitative variables were reported as absolute and relative frequencies and quantitative variables as medians and ranges, after verification of the normality assumption with the Shapiro-Wilk test. Comparison between groups according to the severity of BPD was performed using the χ2 test and Fisher's exact test for qualitative variables and the Kruskal-Wallis test for quantitative variables; a p value < 0.050 was considered significant. Statistical analyses were performed in STATA v.14.
This project was approved by the Institutional Ethics Committee and was carried out in accordance with the guidelines established in the Declaration of Helsinki and local regulations.25
Results
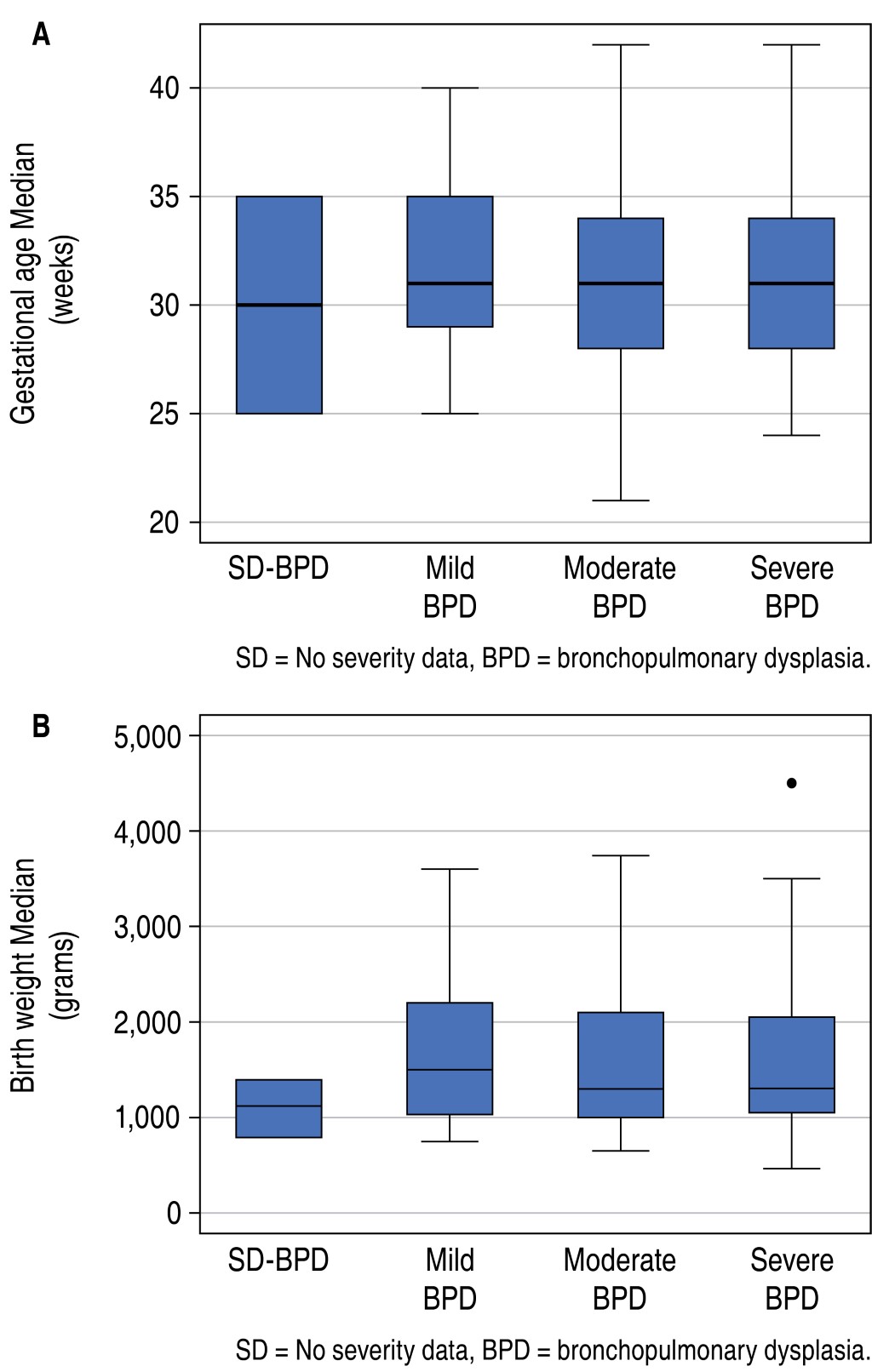
We retrospectively analyzed 386 clinical histories of patients with a diagnosis of BPD who met the inclusion criteria and attended a Pneumology consultation. Three patients were not included in the analysis, one because of cyanotic congenital heart disease and two because of incomplete clinical history. 57.51% (n = 222) were male, with a median gestational age of 31 weeks (range: 28 to 35 weeks), 73.83% (n = 282) of the patients were born before 34 weeks of gestation and had a median birth weight of 1,305 g (range: 1,016 to 2,087 g) (Table 2).
Patients were classified according to the severity of BPD into mild, moderate and severe according to the time of supplemental oxygen use and use of positive airway pressure, 47.15% (n = 182) of the cases were classified as moderate BPD, 33.68% (n = 130) as severe BPD and 18.39% (n = 71) as mild BPD (Table 2).
In all three BPD categories, the most affected were male patients (p = 0.153); median gestational age ranged from 31 to 31.4 weeks, p = 0.450 (Figure 1A); and median birth weight was 1,500 g (range: 1,030 to 2,200 g) in mild BPD group; 1,300 g (range: 1,000 to 2,100 g) in moderate BPD group and 1,305 (range: 1,050 to 2,050 g) in severe BPD group (p = 0.775) (Figure 1B).
The main characteristics of the study population are shown in Table 2. The number of days of oxygen use was higher in the severe BPD group (180 days; range: 96 to 370 days), compared with the mild BPD group (40 days; range: 30 to 57 days), p = 0.001. In the mild and moderate BPD groups, most patients (92.96 and 42.31%, respectively) used oxygen for a shorter period of time (less than three months); while in the severe BPD group, 46.15% used it for periods longer than six months.
Mechanical ventilation was used in the mild and moderate BPD groups for a period of less than one month, compared to the severe BPD group, where 71.54% used it for a period close to two months. Specifically, the use of mechanical ventilation was 12 days (range: 6 to 22) for the mild group, 20 days (range: 10 to 30 days) for the moderate and 54.2 days (95% CI: 49.23 to 59.33 days) for the severe, p = 0.000.
The use of surfactant was established in only 28.45% (n = 109) of the population, and its administration was higher in the severe BPD group (33.08%). On the other hand, at hospital discharge from the newborn unit, a high number of patients with moderate (64.84%) and severe BPD (71.54%) used oxygen, compared to the mild BPD group (25.35%), p = 0.000.
The analysis of variables related to newborn history showed that low birth weight (9.97%), perinatal asphyxia (25.39%), intraventricular hemorrhage (19.69%) and patent ductus arteriosus (PDA) (19.69%) were the most prevalent, being more frequent in the severe BPD group, with no differences found between BPD groups.
The mothers of patients with BPD were mostly (79.27%) between 20 and 39 years of age with a grade of schooling mainly primary education (47.41%); only 3.37% (n = 13) had multiple pregnancies, 26.68% (n = 103) had early rupture of membranes and 15.54% (n = 60) suffered gestational hypertension. 73.63% (n = 282) had cesarean delivery, but no difference was found between BPD groups with respect to vaginal delivery (p = 0.876).
Family history of atopy (familial asthma) and environmental history (exposure to passive smoking, wood smoke and zoonosis) were also evaluated, but none of them showed differences between BPD severity groups (Table 2).
The most frequent comorbidities were: neurological alteration (19.69%) basically due to neurodevelopmental delay, cerebral palsy and central nervous system malformation, among others; cardiac alteration (9.33%), diagnosed by echocardiogram, included mainly PDA, atrial septal defect, ventricular septal defect and pulmonary hypertension (5.18%). Neurological disturbance and pulmonary hypertension were more frequent in the severe BPD group, and showed differences between BPD groups (p = 0.034 and p = 0.037 respectively); recurrent wheezing was present in only 0.78% of the population.
Information on other comorbidities found is detailed in Table 2. None of the patients evaluated (n = 383) were found to have diaphragmatic hernia. Analysis by BPD severity subgroups showed no differences.
The number of hospitalizations and medical care received in the first two years of life documented within the study period was evaluated, as well as signs and symptoms presented at the first Pneumology consultation. 95.34% of the patients had no or a maximum of three hospitalizations, while 4.66% had between four and seven hospital admissions. Regarding the number of consultations, 89.38% of the patients had between one and 10 Pneumology consultations (median: 4 consultations; range: 2 to 7 consultations), 8.81% between 11 and 20 consultations and 1.81% between 21 and 30 consultations and no differences were observed between BPD groups (p = 0.707), the details by severity groups can be seen in Table 3.
The mean age of the first consultation with Pneumology was 6.69 months (range: 3.78 to 15.51 months), and there was no significant difference in relation to the severity of BPD (p = 0.141).
Of the patients, 69.17% were symptomatic at the first consultation (Table 3) and 26.94% used oxygen, with the frequency of use being higher the greater the severity of BPD (p = 0.000). Chest X-ray was ordered in all patients in their first outpatient control, and analyzed in conjunction with the Radiology Service, finding results compatible with BPD such as linear interstitial infiltrates, reticular and hyperinflation in a total of 143 patients 37.05%; no differences were evident between severity groups (p = 0.353).
The use of medicines such as prenatal corticosteroid (18.60%), postnatal inhaled corticosteroid (80.31%), diuretics (58.29%) and salbutamol (39.90%) were part of the therapeutic scheme received by these patients. All medications had frequencies of use that increased gradually according to the severity of BPD, but only inhaled corticosteroid (p = 0.015) and salbutamol (p = 0.014) showed differences between groups.
Discussion
The present study, based on information from a pediatric population of patients referred from early neonatal care in our institution and from patients referred from external hospitals, generates a great diversity in the population group attended, becoming a good option to describe factors associated with the risk of suffering BPD, as well as comorbidities characteristic of children with this disease.
Moderate and severe forms of BPD were frequent in the study population, especially in patients born around week 31 and with low birth weight (1,500 g), coinciding with that reported in the study by D'Angio et al.26 who demonstrated that premature, small for gestational age or intrauterine growth restricted infants have a higher risk of adverse pulmonary effects and worse complications.
Lum et al.27 report that children with a history of BPD are at increased risk of childhood respiratory symptoms or disease and chronic hypoxemia, due to decreased airway caliber, decreased expiratory flows and volumes, and reduced diffusing capacity reflecting disrupted alveolar development, decreased surface area for gas exchange, and disrupted angiogenesis. This work found that the requirement for mechanical ventilation, the number of days of prolonged supplemental oxygen use, as well as the use of oxygen at hospital discharge are factors that in our population fit the diagnostic criteria for BPD, which are usually necessary interventions in the most critical stages of neonates and are clearly related to the pathogenesis of the disease, as described in the study by Tapia et al.28
BPD was more prevalent in male patients and in those who presented risk factors such as intraventricular hemorrhage, PDA and enterocolitis in the neonatal stage, showing an increase related to the severity of BPD, although it was not statistically significant. There are some factors that have been frequently identified in the development of BPD, among which are gestational age, male sex, and PDA.
In contrast to the study by Cunha et al.29 regarding the characteristics of mothers of BPD patients, preeclampsia was not found to be a variable of interest, while premature rupture of membranes was, as was the study by Cokyaman et al.30 The high frequency of cesarean section observed in our investigation agrees with that reported by Cunha et al.29 and allows us to infer that these women probably had an early diagnosis of maternal and fetal complications that could have led them to a more rigorous control of pregnancy and delivery, despite their low schooling. Maternal age, unlike that reported by Klinger et al. was not related to the presence or severity of BPD in newborns with low birth weight.31
According to Cherian et al,32 oxygen is the most commonly used therapy during the stay of cases in neonatal units and plays an important role, since hypoxia can lead to pulmonary vasoconstriction and pulmonary hypertension, while hyperoxia can lead to the development of BPD, retinopathy of prematurity or injury to the cerebral white matter; the latter injury may be associated with neurodevelopmental delay (characteristic of these patients), referred to in our publication as neurological impairment.
The long-term repercussions commonly present are chronic pulmonary alterations that lead to frequent hospitalizations, generating up to 49% of readmissions during the first year of life.33 In our study, during the first two years of life, the vast majority of cases required at least three hospitalizations, between one and 10 consultations by Pneumology and first attention by our service at around six months of age.
Other alterations that have been frequently identified in the development of BPD are persistent anomalies in the development of pulmonary function with the presence of chronic cough, wheezing and the use of bronchodilator medicines, with a high incidence of asthma at five years of age.33-35 In our study, although cough and wheezing were documented, we did not find a high frequency in the patients seen in consultation, but the use of bronchodilators did have an important frequency, their formulation being significant in patients with BPD.
In our study, inhaled corticosteroids were used with a high frequency and we were able to demonstrate that their use in the management of BPD is very useful. The above is aligned with that reported by the neurosis study, a double-blind, placebo-controlled trial conducted in 40 centers in nine European countries that measured the long-term effect of inhaled corticosteroids in 863 preterm infants aged 23 to 27 weeks, finding that the incidence of BPD was 27, 8% compared to 38% in those who did not receive this therapy, and also showed that in the long term there was no neurodevelopmental disability, deafness or blindness,36,37 and therefore they recommend its use, given its anti-inflammatory activity and fewer side effects than systemic steroids.36
In general, the data obtained show similar frequencies in the maternal and newborn variables reported in the study by Maya-Barrios et al.33 conducted in the Mexican neonatal population.
One of the limitations of the study, given its retrospective nature, was the inability to perform reliable statistical estimation of important variables in patients with BPD, such as the use of surfactant and the presence of gastrointestinal comorbidity. This was due to the fact that it was not possible to establish with certainty in the entire population the use of surfactant and the performance of tests to confirm the presence of gastroesophageal reflux and/or alterations in swallowing mechanics, which are part of the gastrointestinal alterations. This may be due to underreporting in the medical records.
The goal with these patients has been to achieve increasingly faster discharges so that they are incorporated as soon as possible to an adequate outpatient follow-up that includes management by a multidisciplinary team that leads to the prevention of respiratory diseases, to achieve an early withdrawal of home oxygen and to an adequate nutritional, cardiovascular and neurodevelopmental follow-up, for which we have been working institutionally in the constitution of the BPD clinic with the support of different services.
Conclusions
The population of patients with BPD that was part of this study was characterized by having mainly severe and moderate disease requiring prolonged use of mechanical ventilation and oxygen therapy. No maternal, newborn history, or environmental factors were found to be statistically associated with the severity of BPD. Neurological alterations and pulmonary hypertension were significant complications in our population. Despite the severity of BPD, there was no high requirement for hospitalization, achieving outpatient follow-up through outpatient consultation in the Pneumology Service, where the use of inhaled corticosteroid proved to be very useful.
Acknowledgements
Horacio Márquez González, for his advice in the design of the study and Sandra Johanna Echeverry Coral for the statistical analysis and methodological advice.
AFILIACIONES
1Hospital Infantil de México "Federico Gómez", Mexico City, Mexico.Conflict of interests: The authors declare that they have no conflict of interests with respect to the research, authorship or publication. The authors have received no funds or benefits from industry or elsewhere to carry out this study.
REFERENCES
Baud O, Maury L, Lebail F, Ramful D, El Moussawi F, Nicaise C, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet [Internet]. 2016 Apr 30 [cited 2021 Dec 23];387(10030):1827-1836. Available in: https://linkinghub.elsevier.com/retrieve/pii/S0140673616002026
Instituto Mexicano del Seguro Social. Prevención, Diagnóstico y Tratamiento de la Displasia Broncopulmonar en niñas/niños menores de 2 años en el segundo y tercer nivel de atención. [Internet]. Catálogo Maestro de Guías de Práctica Clínica: 2015. p. IMSS-776-15. Available in: http://www.cenetec-difusion.com/CMGPC/IMSS-776-15/ER.pdf
D'Angio CT, Ambalavanan N, Carlo WA, McDonald SA, Skogstrand K, Hougaard DM, et al. Blood cytokine profiles associated with distinct patterns of bronchopulmonary dysplasia among extremely low birth weight infants. J Pediatr [Internet]. 2016;174:45-51.e5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2982806/pdf/nihms248679.pdf
Tapia JL, Agost D, Alegria A, Standen J, Escobar M, Grandi C, et al. Bronchopulmonary dysplasia: Incidence, risk factors and resource utilization in a population of South American very low birth weight infants. J Pediatr (Rio J). 2006;82(1):15-20. Available in: http://www.jped.com.br/ArtigoDetalhe.aspx?varArtigo=1431&idioma=pt-BR
Giubergia V, Bauer G, Rentería F, González H, Vila F, Giubergia V, Michelini A, Aguerre V, et al. Seguimiento neumológico de los niños con displasia broncopulmonar al alta de la Unidad de Cuidados Intensivos Neonatal Parte 1: Epidemiología, fisiopatología y clínica. Arch Argent Pediatr. 2013;111(2):165-172.
|
Table 1: Classification of bronchopulmonary dysplasia. |
||
|
Gestational age at birth |
< 32 weeks |
> 32 weeks |
|
Time of evaluation |
36 weeks postconceptional age or hospital discharge (whichever comes first) |
More than 28 days but less than 56 days postnatal age or hospital discharge (whichever occurs first) |
|
Oxygen treatment |
More than 21% during 28 days or more |
|
|
Mild BPD |
Breathes room air at 36 weeks postconceptional age or at discharge (whichever comes first) |
Breathing room air at postnatal day 56 or discharge (whichever comes first)
|
|
Moderate BPD |
Receives supplemental oxygen with FiO2 2 < 30% at 36 weeks postconceptional age or at discharge (whichever occurs first) |
Receives supplemental oxygen with FiO2 < 30% at postnatal day 56 or at discharge (whichever occurs first) |
|
Severe BPD |
Receives supplemental oxygen with FiO2 ≥ 30% and/or CPAP or MV at 36 weeks postconceptional age or at discharge (whichever occurs first) |
Receives supplemental oxygen with FiO2 ≥ 30% and/or CPAP or MV at postnatal day 56 or at discharge (whichever occurs first) |
|
CPAP = continuous positive airway pressure, BPD = bronchopulmonary dysplasia, FiO2 = fractional inspired oxygen, MV = mechanical ventilation. Adapted from: Jobe A, et al.3 |
||
|
Table 2: Main characteristics of the study population. |
|||||
|
|
BPD Severity |
||||
|
SD |
Mild n (%) |
Moderate n (%) |
Severe n (%) |
p |
|
|
Variables |
3 |
71 (18.39) |
182 (47.15) |
130 (33.68) |
NA |
|
Maternal variables |
|||||
|
Sex Female Male |
1 |
26 (36.62) 44 (61.97) |
79 (43.41) 103 (56.59) |
55 (42.31) 75 (57.69) |
0.153 |
|
Gestational age Median and range (weeks) Range |
|
31.0 29-35 |
31.4 28-34 |
31.2 28-34 |
0.861 |
|
Birth weight Median (g) Range |
|
1,500 1,030-2,200 |
1,300 1,000-2,100 |
1,305 1,050-2,050 |
0.597 |
|
Days with O2 Median (days) Range |
|
40 30-57 |
107 63-210 |
180 96-370 |
0.001* |
|
Mechanical ventilation days Median (days) Range |
36 |
12 6-22 |
20 10-30 |
54 49-59 |
0.001* |
|
Surfactant Yes |
|
17 (23.94) |
49 (26.92) |
43 (33.08) |
NC |
|
Egress with O2 Yes |
|
18 (25.35) |
118 (64.84) |
93 (71.54) |
0.000* |
|
Prenatal history |
|||||
|
Chorioamnioitis Yes |
|
0 (0) |
3 (1.65) |
2 (1.54) |
0.717 |
|
Intraventricular hemorrhage Yes |
|
9 (12.68) |
31 (17.03) |
27 (20.77) |
0.344 |
|
Persistent ductus arteriosus Yes |
|
10 (14.08) |
33 (18.13) |
33 (25.38) |
0.115 |
|
Enterocolitis Yes |
|
4 (5.63) |
20 (10.99) |
13 (10.00) |
0.466 |
|
Perinatal asphyxia Yes |
|
17 (23.94) |
44 (24.18) |
37 (28.46) |
0.577 |
|
Malnutrition at birth Yes |
|
66 (92.96) |
165 (90.66) |
122 (93.85) |
0.285 |
|
Maternal background |
|
|
|
|
|
|
Maternal age (years) < 20 20-39 > 40 |
|
10 (14.08) 58 (81.69) 3 (4.23) |
28 (15.38) 141 (77.47) 13 (7.14) |
13 (10.00) 106 (81.54) 11 (8.46) |
0.574 |
|
Maternal schooling Primary High school Technical University Does not read or write |
|
31 (43.66) 28 (39.44) 2 (3) 7 (10) 3 (4) |
94 (51.65) 68 (37.36) 4 (2) 16 (9) 0.00 (0.00) |
57 (43.85) 52 (40.00) 4 (3.08) 14 (10.8) 3 (2.31) |
0.39 |
|
Cesarean section Yes |
|
54 (76.06) |
133 (73.08) |
95 (73.08) |
0.876 |
|
Multiple pregnancy Yes |
|
3 (4.23) |
5 (2.75) |
5 (3.85) |
0.807 |
|
Premature rupture of membranes Yes |
|
19 (26.76) |
44 (24.18) |
40 (30.77) |
0.432 |
|
Gestational hypertension Yes |
|
12 (16.9) |
29 (15.93) |
19 (14.62) |
0.905 |
|
Family and environmental background |
|||||
|
Passive smoking Yes |
|
10 (14.08) |
26 (14.29) |
12 (9.23) |
0.493 |
|
Zoonoses Yes |
|
22 (30.99) |
48 (26.37) |
26 (20.00) |
0.338 |
|
Wood smoke Yes |
|
4 (5.63) |
4 (2.2) |
4 (3.08) |
0.393 |
|
Family history of asthma Yes |
|
5 (7.04) |
7 (3.85) |
9 (6.92) |
0.462 |
|
* Statistically significant. BPD = bronchopulmonary dysplasia. |
|||||
|
Table 3: Characteristics of medical care. |
|||||
|
|
BPD Severity |
||||
|
SD |
Mild n (%) |
BPD Severity Moderate n (%) |
Severe n (%) |
p |
|
|
Variables |
3 |
71 (18.39) |
182 (47.15) |
130 (33.68) |
|
|
Medical care received |
|||||
|
Hospitalizations due to respiratory causes 0 to 3 4 to 7 |
|
68 (95.77) 3 (4.23) |
177 (97.25) 5 (2.75) |
120 (92.31) 10 (7.69) |
0.140 |
|
Number of pulmonology consultations |
|||||
|
Median and range Range 0 to 10 11 to 20 21 to 30 |
|
5 (2 to 7) 61 (85.92) 8 (11.27) 2 (2.82) |
4 (2 to 7) 164 (90.11) 16 (8.79) 2 (1.10) |
5 (2 to 7) 118 (90.77) 10 (7.69) 2 (1.54) |
0.489
0.707 |
|
Characteristics of the first pulmonology consultation |
|||||
|
Age Median (months) Range |
26 |
5.95 2.84 to 12.72 |
6.27 3.22 to 15.35 |
6.88 4.60 to 15.61 |
0.141 |
|
Weight Median (kg) Range |
19 |
5.0 3.5 to 8.0 |
4.8 3.5 to 7.2 |
4.7 3.29 to 7.1 |
0.446 |
|
Size Medium (cm) Range |
20 |
60 53 to 71 |
59 52 to 72 |
57 51 to 70 |
0.590 |
|
Nasal obstruction Yes |
|
3 (4.23) |
10 (5.49) |
4 (3.08) |
0.641 |
|
O2 saturation Median (%) Range |
19 |
94 93 to 95 |
93 91 to 95 |
93 92 to 96 |
0.269 |
|
Rhinorrhea Yes |
|
3 (4.23) |
15 (8.24) |
9 (6.92) |
0.604 |
|
Wheezing Yes |
|
3 (4.23) |
5 (2.75) |
8 (6.15) |
0.305 |
|
Pulling Yes |
|
1 (1.41) |
5 (2.75) |
7 (5.38) |
0.315 |
|
Cough Yes |
|
14 (19.72) |
32 (17.58) |
30 (23.08) |
0.493 |
|
Uses O2 Yes |
|
9 (12.68) |
45 (24.73) |
49 (37.69) |
0.000* |
|
Cyanosis Yes |
|
2 (2.82) |
4 (2.20) |
5 (3.85) |
0.668 |
|
Crypts Yes |
|
4 (5.63) |
7 (3.85) |
10 (7.69) |
0.330 |
|
Dyspnea Yes |
|
4 (5.63) |
3 (1.65) |
7 (5.38) |
0.115 |
|
* Statistically significant. BPD = bronchopulmonary dysplasia. |
|||||


