Polyunsaturated fatty acids and their derivatives regulate respiratory infections
Ruiz, Andy1,2; Romero-García, Aida Susana2; Mancilla-Jiménez, Raúl2; Juárez, Esmeralda1
Ruiz, Andy1,2; Romero-García, Aida Susana2; Mancilla-Jiménez, Raúl2; Juárez, Esmeralda1
ABSTRACT
The regulation of inflammation is a complex pathophysiological process that depends on the production of oxygenated lipid derivatives essential polyunsaturated fatty acids, like omega-3 and omega-6, among which are the lipoxins resolvins and protectins, called specialized pro-resolving lipid mediators (SPM). Their activity is associated with the control of respiratory infection processes to modulate the production of proinflammatory cytokines, avoiding damage due to inflammation-associated necrosis, reducing microbial loads, and promoting tissue remodeling. Therefore, we review some of the biochemical, physiological and immunological aspects of SPM in the regulation of inflammation in respiratory infections.KEYWORDS
Eicosapentaenoic acid, docosahexaenoic acid, inflammation, respiratory infections, specialized pro-resolving lipid mediators.Introduction
Polyunsaturated fatty acids (PUFA), such as omega-3, are obtained from rich sources of fish, salmon, walnuts and flaxseeds, while rich sources of omega-6 include vegetable oils from corn, safflower, sunflower, soybean and some animal products.1-3
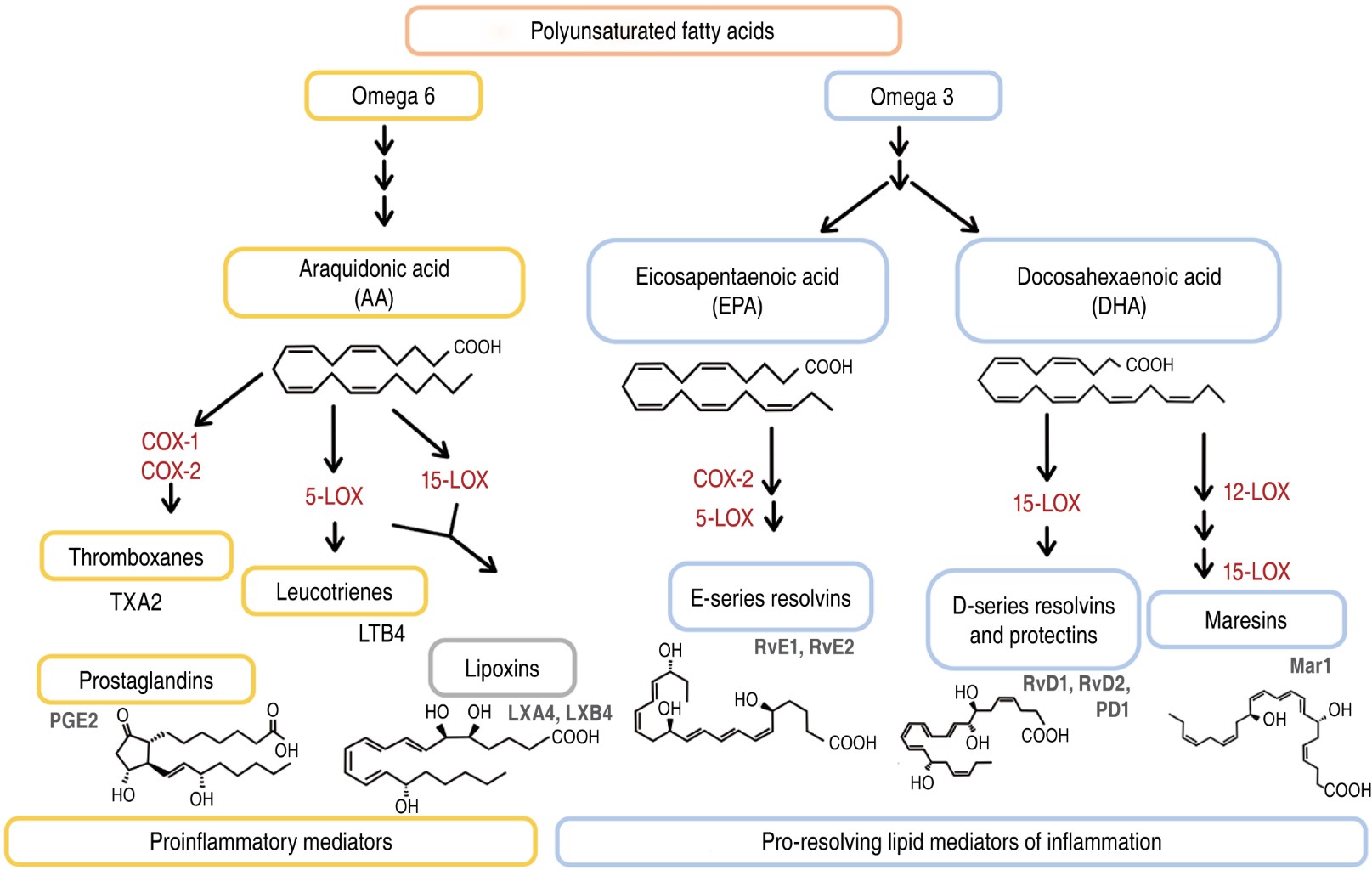
PUFA have been increasingly studied for their involvement in the regulation of inflammatory responses, such as the production of specialized pro-resolving lipid mediators (SPMs). PUFA-derived SPMs such as linoleic acid (C18: Δ2, n-6), arachidonic acid (AA, C20: Δ4, n-6), eicosapentaenoic acid (EPA, 20:55,8,11,14,17) and docosahexaenoic acid (DHA, 22: 64,7,10,13,16,19) are generated from enzymatic reactions mediated by lipooxygenases (LOX) and/or cyclooxygenases (COX), which include DHA-derived protectins and D-series resolvins, EPA-derived E-series resolvins, and AA-derived lipoxins, as shown in Figure 1.4-9
In both in vitro and in vivo models, SPMs promote bacterial clearance by stimulating the production of antimicrobial peptides,7,10 increase the phagocytic activity of macrophages11-13 and decrease the production of proinflammatory cytokines. In addition, they aid in tissue repair, increase host defenses and improve survival.14,15
There is evidence that respiratory infections are affected by the patient's nutritional status, metabolic status, medication, complications and the course of the pulmonary disease itself,16-22 so achieving a balance between the protective and detrimental effects of the immune response may help to reduce morbidity and mortality and complications in respiratory infections. Therefore, investigating the biochemical, immunological and pathophysiological aspects of PUFA and their derivatives will help to envision routes, routes of administration and nutritional formulations that will help to select strategies to eradicate respiratory tract pathogens.
Physiological role of PMS
The biological actions of SPMs are mediated by the activation of cognate receptors. Signaling is initiated locally by specific G protein-coupled receptors (GPCRs) that are expressed in different cell types (polymorphonuclear cells [PMN], dendritic cells, monocytes, macrophages, epithelial cells, fibroblasts, adipocytes, etc.) and promote tissue selectivity, exerting their action against extracellular responses. Table 1 shows some of the SPM receptors found to date, as well as their agonists, antagonists and regulatory genes.23-30
ALX was the first receptor identified, it is activated by cognate endogenous ligands, including lipoxin A4 (LXA4) and resolvins D1 and D3 (RvD1 and RvD3), as well as those triggered by aspirin (AT-LXA4, AT-RvD1). RvD1 activates the GPR32 receptor that leads to the regulation of several micro-RNAs (miRNAs) involved in the orchestration of acute inflammation, including miR-(miRNA)146b, miR-208a and miR-219. This receptor also mediates the biological actions of RvD5 in the context of bacterial infections, whereby its activation by RvD5 leads to enhanced bacterial phagocytosis in human macrophages and downregulation of several proinflammatory genes, including NF-κB (nuclear factor enhancer of activated B-cell kappa light chains) and TNF-α (tumor necrosis factor alpha).31,32
The biological effect of resolvins is mediated by ALX, FPR2, DRV1, GPCR32, DRV2, GPCR18, ChemR23 or ERV1 receptors. RvD1 has been shown to inhibit canonical NF-κB (p65/p50) and activation of the non-canonical NF-κB pathway (p50/p50), leading to inhibition of apoptosis and blockade in the production of proinflammatory cytokines, reducing PMN transendothelial migration, increasing macrophage activity, resulting in clearance of apoptotic cells.33 Moreover, RvD1 is able to activate PPARγ (peroxisome proliferator-activated receptor gamma) and suppress NF-κB degradation via p65.34
Some studies have shown that RvD2 activates the DRV2/GPCR18 receptor controlling phagocyte functions in both humans and mice for these receptors, where bacterial infections were controlled, improving survival in murine and providing organ protection, while these actions were diminished in DRV2 knockout (KO) transgenic mice.35
In the case of RvE1, it has been shown to function as an agonist for ChemR23/ERV and an antagonist for the LTB4 receptor (BLT1) in PMN. Being able to inhibit PMN superoxide anion in response to TNF-α or bacterial peptide N-formyl-methionyl-leucyl-phenylalanine (f-MetLeuPhe), it also stimulates phagocytosis of apoptotic PMN by macrophages. While in a rabbit model of periodontitis, administration of RvE1 resulted in regeneration of damaged tissues, including bone, compared to the use of aspirin or steroids such as dexamethasone, it selectively inhibited thromboxane, demonstrating its ability to exert anti-inflammatory effects.26
Evaluations of SPM concentrations in the body are performed using high structural resolution techniques such as liquid chromatography-mass spectrometry (LC-MS), metabololipidomics and UV spectroscopy. Data reported to date suggest that the basal levels of SPMs are in the submicromolar and nanomolar ranges.23,29,30,34,36,37 Shivakoti et al.38 conducted a comparative study of the concentrations of some SPMs, where they determined that Australian diabetic (DM) patients had higher serum concentrations of RvD1, RvD2, RvE1, RvE2 and Mar1 compared to patients with tuberculosis (TB) and patients with TB and diabetes (TB-DM), indicating that infection promotes an imbalance between these lipid mediators, giving rise to the consideration of SPM levels as biomarkers of disease.
PMS in respiratory diseases infectious and non-
The human respiratory system is usually divided into upper and lower respiratory tract. The upper airways include nasal cavities, oral cavity, paranasal sinuses, nasopharynx and larynx (which play an important role in particle clearance). The lower airways include the trachea, main bronchi, terminal bronchi, and respiratory bronchi, as well as the alveoli.39,40 Infections can affect both airways, the most common being acute rhinopharyngitis (common cold, caused by rhinovirus, coronavirus and respiratory syncytial virus [RSV], and more rarely by enterovirus, influenza and parainfluenza).41-47 In murine models, it has been shown that infection by H5N1 influenza virus causes a deregulation in the expression and signaling of PMS, such as lipoxins,48 while exogenous administration of PD1 inhibits infection by this virus, improving survival and lung function.49 On the other hand, Ramón et al.50 demonstrated a coadjuvant effect with the administration of 17(S)-hydroxydocosahexaenoic acid (17-HDHA) after vaccination against influenza, by significantly increasing the levels of anti-H1N1 antibodies in serum, as well as the number of B cells in murine bone marrow.
Other frequent infections are pharyngotonsillitis (inflammation of the oropharyngeal membranes and palatine tonsils, commonly caused by adenovirus, parainfluenza, Epstein-Barr virus, Coxsackievirus and group A β-hemolytic Streptococcus),43,44,51-53 and rhinosinusitis (inflammation of the mucosa lining the paranasal sinuses, caused by Haemophilus influenzae, Staphylococcus aureus, Staphylococcus pyogenes, Bacteroides sp. and Fusobacterium sp.).51,54,55 In a model of infection with H. influenzae, administration of AT-RvD1 has been found to regulate leukocyte transport to the lung, increasing phagocytosis of neutrophils by macrophages and reducing levels of interleukin 6 (IL-6) and TNF-α.56
On the other hand, the permeability of the alveolar epithelium can trigger an inflammatory response by the entry of different exogenous and endogenous agents that can persistently stimulate the organism, which implies a challenge for the maintenance of homeostasis and the resolution of inflammation.
Some microorganisms have the capacity to become chronically established, such as Mycobacterium tuberculosis, the cause of TB, which has the highest number of deaths due to infectious disease in the world after the human immunodeficiency virus (HIV).57-59 In an experimental model of mice deficient in 5-lipooxygenase (5-LO, an enzyme responsible for the production of lipoxins), it appears to have better control of M. tuberculosis infection compared to wild-type mice infected with M. tuberculosis treated with a 5-LO inhibitor, where the latter had higher mortality and higher bacterial load. These results suggest that infection control is related to leukotriene production (proinflammatory pathway) rather than lipoxin production (anti-inflammatory pathway).60 While in another in vitro model of human macrophages infected with the virulent Mtb H37Rv strain treated exogenously with RvD1 and Mar1 induced the expression of antimicrobial peptides such as BPI (bactericidal permeability-increasing protein) and the human cathelicidin LL37, regulating the production of TNF-α and controlling the intracellular growth of Mtb.10 These investigations show us strategies that may eventually be used to support current TB treatment, either by supplementation of PMS precursors such as DHA/EPA or by exogenous administration of the PMS themselves.
Other external agents that can cause respiratory conditions include allergens (e.g., Derp2 proteins present in dust mite feces), non-degradable particles (such as asbestos) and even endogenous particulate crystals (e.g., cholesterol),61-63 not to mention cigarette smoke, which is associated with chronic respiratory, cardiovascular and tumor diseases, affecting the phagocytic capacity of macrophages.39,64-68 Some research has shown that prostaglandin analogues and lipoxins have physicochemical properties that improve the use of glucocorticoids, since a decrease in the latter improves the adverse effects, as well as resistance to steroids in asthma.69-71 In addition, in a model of allergic asthma, it was determined that the administration of some activators such as TLR7 (toll like receptor 7) increased DHA-derived SPMs such as PD-1, 17-HDHA and 14-HDHA, helping to control the eosinophilia characterized in this animal model, as well as in another model by intraperitoneal administration of RvE1.72,73
Chronic obstructive pulmonary disease (COPD), neonatal respiratory distress syndrome (NRDS), acute respiratory distress syndrome (ARDS), acute lung injury (ALI) and asthma are respiratory system conditions with high incidence, morbidity and mortality. COPD is characterized by airflow limitation and is associated with an abnormal inflammatory response of the lungs to noxious particles or gasses. Tobacco smoke is the main risk factor,74-76 followed by air pollution,77,78 occupational exposure to dust and chemicals, recurrence of respiratory infections during childhood or genetic predisposition. Some studies in murine models have focused on the exogenous administration of LXA4, since this SPM competes with serum amyloid A (SAA, Serum amyloid A) proteins for the GPCR FPRR/ALX, SAA increases considerably in infections and is related to excessive neutrophil recruitment in COPD, therefore, both act as antagonists, which may help prevent chronic inflammation and pulmonary emphysema.75,76,79
On the other hand, NRDS, ARDS and ALI are diseases related to the pulmonary surfactant system, but also occur more frequently in the context of pneumonia, sepsis, aspiration of gastric contents or severe trauma, unlike asthma, which is considered a highly prevalent heterogeneous inflammatory disorder of the airways due to inflammation caused by various allergens.80-82 Eickmeier et al.83 found that administration of AT-RvD1 in a murine model of ALI decreased the amount of bronchoalveolar lavage fluid neutrophils, improved epithelial and endothelial barrier integrity, significantly decreased levels of proinflammatory cytokines such as interleukin 1β (IL-1β), IL-6 and TNF-α, as well as nuclear translocation of p65 phosphorylated by NF-κB, so this SPM could also work for NRDS and ARDS.
Currently, COVID-19 disease caused by the SARS-CoV-2 coronavirus has prompted the search for new therapeutic strategies to combat the severity of the disease, focusing on the elimination of responses exacerbated by the production of proinflammatory cytokines,84 where some groups focused on SPM precursors, such as omega-3 PUFA supplementation, finding improvements in some parameters of respiratory and renal function in critically ill patients with COVID-19 evaluated for one month, compared to patients without supplementation.85 Evaluation of the levels of some PMS in patients diagnosed with COVID-19 showed that critically ill patients had lower concentrations of PMS than those who were discharged.86,87 Recchiuti et al.88 found in an in vitro model of macrophages with or without cystic fibrosis exposed to SARS-CoV-2 virion glycoprotein S (Spike 1) that both RvD1 and RvD2 were able to regulate inflammatory functions by modulating miR-16, miR-29a and miR-103, and simultaneously selectively increased miR-223 and miR-125a, involved in NF-κB activation and macrophage inflammatory polarization. However, it remains to be elucidated whether different disease-associated risk factors including advanced age, hypertension, diabetes, obesity, or other comorbidities have any association with PMS.
As has been seen throughout the text, the analysis of the biological effects of PMS in respiratory infections may lead to new proposals for therapeutic immunomodulation. Recently, De Toledo et al.64 demonstrated that fraction 39 of the mucus of the slug Phyllocaulis boraceiensis contains PUFA with potent antiviral activity against measles virus and influenza virus. Cell viability and toxicity of the mucus were evaluated in Madin-Darby canine kidney cells (MDCK) by the 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazole bromide (MTT) assay, where they demonstrated that hydroxylated PUFA interfered with influenza virus binding to the host cell receptor, causing reduction in viral titers.
Moreover, in an in vitro model of human neutrophils, aspirin-triggered administration of lipoxin (15-epi-LXA4) abrogates the suppression of myeloperoxidase (MPO, an enzyme with microbicidal activity) neutrophil apoptosis by blocking integrin β2-mediated signaling, improving the resolution of MPO-sustained lung injury.89-91 Meanwhile, in a murine model, acute lung injury by intraperitoneal injection of Escherichia coli was evaluated in mice and it was found that subsequent treatment with 15-epi-LXA4 promoted neutrophil apoptosis and improved the resolution of inflammation in lung injury, comparable to mice treated with RvD1 prior to ALI by LPS, where RvD1 improved the survival rate of mice exposed to ALI with inhibition of TNF-α, IL-6 and decreased COX-2 expression.92 Similar results have been found with the administration of RvE1 in a murine model of pneumonia, with exposure to E. coli, where there was a reduction in the production of proinflammatory cytokines, a decrease in PMN and a reduction in E. coli bacterial loads, improving murine survival.11
On the other hand, Raposo et al.93 evaluated the nutritional intake of vitamin C, vitamin E, DHA, AA, selenium and zinc in a cohort of more than 1,500 individuals aged 25 to 64 years who were followed for nine months, found an association in the susceptibility to upper respiratory tract infection in women than in men due to a decrease in the intake of DHA, AA and vitamins C and E. In contrast, in human lung fibroblasts and bronchial cell line (BEAS-2B) it has been shown that PUFA such as DHA, EPA and ALA (α-linolenic) elicit an amplification of inflammatory responses to viral and bacterial components, with production of IL-6 and CXCL8, suggesting that polyunsaturated fatty acids have no anti-inflammatory effects in these lung cells.94 A brief summary of the action of SPMs are shown in Table 2.
Involvement of SPM in the resolution of inflammation
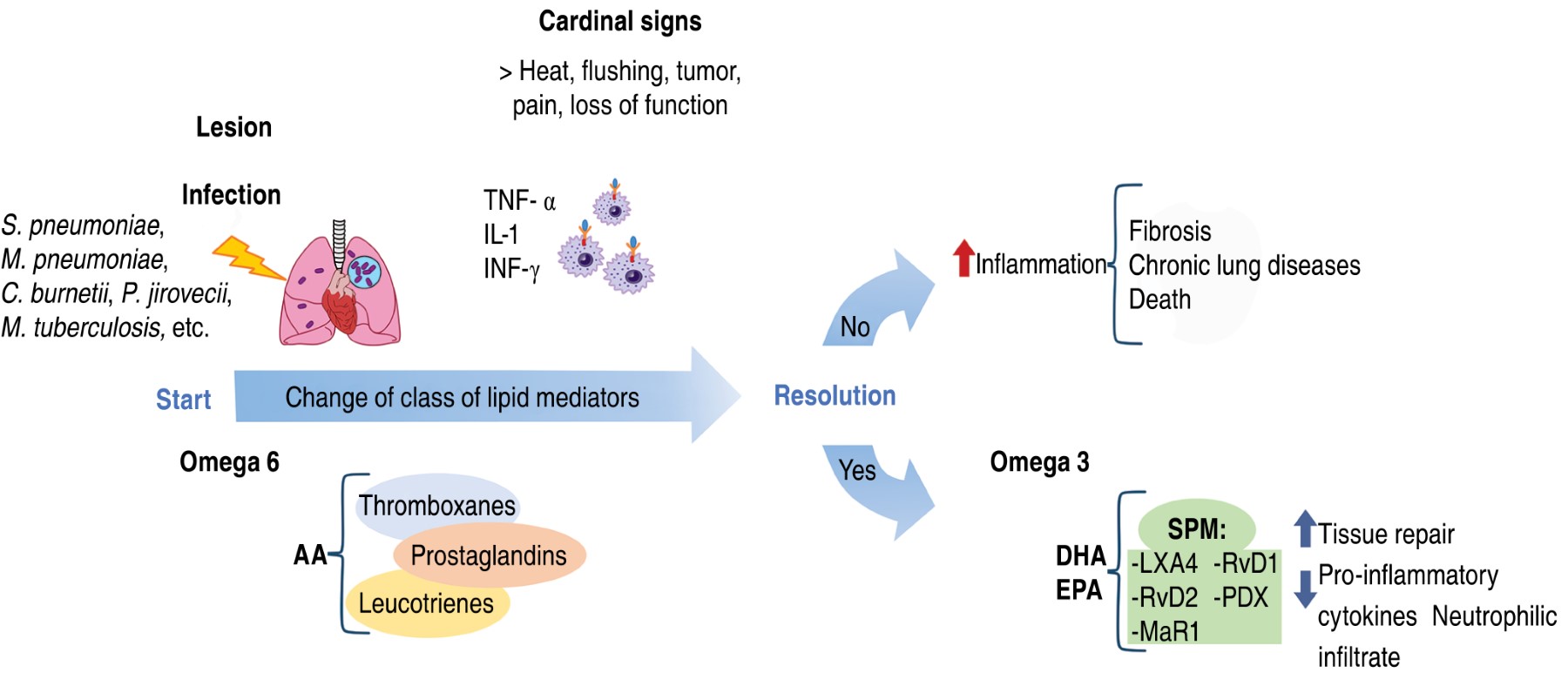
Inflammation is a response of an organism's immune system to damage caused by pathogens or substances of a biological, chemical, physical or mechanical nature and, depending on the duration, can be classified as acute or chronic.
Acute inflammation involves significant changes in plasma levels of histamine, bradykinin, prostaglandins, leukotrienes, thromboxanes and proinflammatory cytokines (TNF-α, IL-1, IL-1β, IL-2 and IL-6), crucial for controlling and eliminating harmful agents,99-104 but if acute inflammation is sustained, it leads to chronic inflammation with systemic and deleterious repercussions for the host, such as tissue infiltration by leukocyte aggregates (granuloma formation), uncontrolled collagen biosynthesis, leading to fibrosis or cirrhosis, permanent loss of normal tissue function (functio laesa) or oxidative damage to deoxyribonucleic acid (DNA), leading to degenerative diseases such as autoimmune diseases, cardiovascular disorders, osteoporosis, rheumatoid arthritis, Alzheimer's disease, certain types of cancer and even death.103
Thus, the involvement of SPMs in the maintenance and response of inflammation is peremptory, performing the switch from a proinflammatory to an anti-inflammatory phenotype, thus aiding in tissue repair and the restoration of homeostasis,105 as shown in Figure 2.104
Conclusions
PUFA and their derivatives, SPM, have a protective and controlling effect on the elimination of pathogenic microorganisms, inflammation and tissue repair, which makes them important candidates for the search for new therapeutic strategies, as well as possible biomarkers. Further knowledge of their signaling mechanisms, synthesis pathways, production of their epimers, and research evaluating PUFA consumption and SPM levels in healthy subjects versus patients with respiratory diseases is needed to better understand the relationship between overall dietary PUFA profiles and their impact on future nutritional or pharmacological interventions as strategies to eradicate pathogens from various respiratory conditions.
Acknowledgments
Andy Ruiz thanks the Doctoral Program in Biological Sciences of the UNAM, as well as the National Council of Science and Technology (CONACyT) for the scholarship received during his studies and acknowledges the comments made by Dr. José Ángel Santiago Terrones.
AFILIACIONES
1Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City, Mexico 2Universidad Nacional Autónoma de México, Mexico City, Mexico.Conflict of interests: The authors declare that they have no conflict of interests.
REFERENCES
Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Fatty acids as biocompounds: Their role in human metabolism, health and disease - a review. part 1: Classification, dietary sources and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155(2):117-130. Available in: https://doi.org/10.5507/bp.2011.038
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al.; and GLIM Core Leadership Committee, GLIM Working Group. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. J Cachexia, Sarcopenia Muscle. 2019;10(1):207-217. Available in: https://doi.org/10.1002/jcsm.12383
Polus A, Zapala B, Razny U, Gielicz A, Kiec-Wilk B, Malczewska-Malec M, et al. Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochim Biophys Acta. 2016;1861(11):1746-1755. Available in: https://doi.org/10.1016/j.bbalip.2016.08.005
Guzmán-Beltrán S, Carreto-Binaghi LE, Carranza C, Torres M, Gonzalez Y, Muñoz-Torrico M, et al. Oxidative stress and inflammatory mediators in exhaled breath condensate of patients with pulmonary tuberculosis. a pilot study with a biomarker perspective. Antioxidants. 2021;10(10):1572. doi: 10.3390/antiox10101572.
Abdillahi SM, Tati R, Nordin SL, Baumgarten M, Hallgren O, Bjermer L, et al. The pulmonary extracellular matrix is a bactericidal barrier against Haemophilus influenzae in chronic obstructive pulmonary disease (COPD): Implications for an in vivo innate host defense function of collagen VI. Front Immunol. 2018;9:1988. doi: 10.3389/fimmu.2018.01988.
Doaei S, Gholami S, Rastgoo S, Gholamalizadeh M, Bourbour F, Bagheri SE, et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: a randomized clinical trial. J Transl Med. 2021;19(1):128. doi: 10.1186/s12967-021-02795-5.
|
Table 1: Receptors, genes and agonists of specialized pro-resolving lipid mediators in various cells. |
||||
|
SPM |
GPCR Receptors |
Gen |
Antagonist |
Cells |
|
RvD1 |
ALX, ALX/FPR2, FPR2, DRV1, GPCR32/ALX |
GPCR32 |
– |
PMN, DC, monocytes, macrophages, macrophages, epithelial cells, fibroblasts |
|
RvD2 |
DRV2, DRV/GPCR2, DRV2/GPCR18, GPCR18 |
– |
– |
NKs, PMNs, lymphocytes, monocytes, macrophages, epithelial cells |
|
RvD3 |
ALX, DRV1 |
– |
– |
Lymphocytes, PMNs, monocytes, macrophages |
|
RvD5 |
DRV1, DRV1/GPCR32 |
GPCR32 |
– |
PMN |
|
AT- RvD1 |
ALX/FPR2 |
– |
– |
NKs, PMNs, lymphocytes, monocytes, macrophages, epithelial cells |
|
RvE1 |
ChemR23, ERV |
CMKLR1 |
BLT1 |
PMN, monocytes, macrophages |
|
RvE2 |
ERV1/ChemR23 |
CMKLR1 |
BLT1 |
Monocytes, macrophages |
|
LXA4 |
ALX, FPR2 |
FPR2 |
CB1 |
NKs, PMNs, lymphocytes, monocytes, macrophages, epithelial cells |
|
AT- LXA4 |
ALX, DRV1/GPCR32 |
GPCR32 |
– |
NKs, PMNs, lymphocytes, monocytes, macrophages, epithelial cells |
|
Mar1 |
– |
– |
BLT1 |
PMNs, lymphocytes, macrophages |
|
SPM = specialized pro-resolving lipid mediators; GPCR = G protein-coupled receptors; RvD1 = resolvin D1; ALX = lipoxin receptor; FPR2 = N-formyl peptide receptor 2; PMN = polymorphonuclear cells; GPCR32 = G protein-coupled receptor 32; DC = dendritic cells; RvD2 = resolvin D2; DRV2 = resolvin D2 receptor; DRV = resolvin D-series resolvin receptor; GPCR2 = G protein-coupled receptor 2; GPCR18 = G protein-coupled receptor 18; NK = natural killer cells; RvD3 = resolvin D3; DRV1 = resolvin D1 receptor; RvD5 = resolvin D5; AT-RvD1 = aspirin-triggered resolvin D1; RvE1 = resolvin E1; ERV = E-series resolvin receptor; CMKLR1 = chemerin chemokine-like receptor 1; RvE2 = resolvin E2; ERV1 = resolvin E1 receptor; LXA4 = lipoxin A4; CB1 = cannabinoid receptor type 1; AT-LXA4 = aspirin-triggered lipoxin A4; Mar1 = maresin 1. |
||||
|
Table 2: Action of specialized pro-resolving lipid mediators in different experimental models. |
|||
|
SPM |
Cell or study model |
Action |
References |
|
Mar1 |
Bronchial epithelial cells |
Reduced IL-6, TNF-α and IL-8, decreased neutrophil accumulation |
13 |
|
|
Human macrophages |
Induces BPI expression, regulates TNF-α production and induces intracellular growth control of Mycobacterium tuberculosis |
10 |
|
AT-RvD1 |
Bronchial epithelial cells |
Modulates LPS-induced bronchoalveolar lavage cell activation and the immune response of Dermatophagoides pteronyssinus mites |
95 |
|
RvE1 |
Murine models of pneumonia |
Reduces IL-1β, IL-6, PMN infiltration, improves survival and decreases bacterial loads |
11 |
|
|
Murine models of critical illness |
Inhibits translocation and activation of NF-κB (p65) |
96 |
|
RvD1 |
Murine model
Murine model
Human alveolar macrophages
Human macrophages |
In Escherichia coli and Staphylococcus aureus infections, it limits PMN infiltration, aids bacterial clearance and enhances PMN infiltration, helps bacterial clearance and increases the resolution of the infection In mice exposed long-term to cigarette smoke, it reduced emphysema and airspace enlargement, as well as and airspace enlargement as well as inflammation, oxidative stress and cell death In human alveolar macrophages from COPD and non-COPD patients decreased IL-6 and TNF-α levels, while increased phagocytosis and promoted an M2 macrophage phenotype Induces BPI and LL37 expression, upregulates TNF-α production and induces intracellular growth control of Mycobacterium tuberculosis |
97
12
68,98
10 |
|
PD1 |
Human eosinophils |
Patients with PD1 impairment contribute to severe asthmatic persistence and severity of the disease, decreased adhesion molecules (CD11b and L-selectin), decreased chemotaxis |
96 |
|
LXA4 |
Serum and murine models |
Negatively regulate protective Th1 lymphocyte responses against Mycobacterium tuberculosis infection |
14 |
|
DHA, EPA and ALA |
Human pulmonary fibroblasts and bronchial cell line (BEAS-2B) |
They cause an amplification of inflammatory responses to viral and bacterial components, with production of IL-6 and CXCL8. |
15 |
|
SPM = specialized pro-resolving lipid mediators of inflammation; Mar1 = maresin 1; IL-6 = interleukin 6; TNF-α = tumor necrosis factor alpha; IL-8 = interleukin 8; BP1 = bactericidal/permeability-increasing protein; AT-RvD1 = aspirin-triggered resolvin D1; LPS = lipopolysaccharide; RvE1 = resolvin E1; IL-1β = interleukin 1β; PMN = polymorphonuclear cells; NF-κB = nuclear factor enhancer of activated B cell kappa light chains (nuclear factor-κB); RvD1 = resolvin D1; COPD = chronic obstructive pulmonary disease; LL37 = cathelicidin; PD1 = protectin D1; LXA4 = lipoxin A4; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; ALA = α-linolenic acid; CXCL8 = chemokine [C-X-X motif] ligand 8. |
|||


