Radiological features of non-neoplastic lesions of mediastinum
Hernández-Morales, Aloisia Paloma1; Jiménez-López, Jolenny1; Sotelo-Robledo, Roberto1; Juárez-Hernández, Fortunato1
Hernández-Morales, Aloisia Paloma1; Jiménez-López, Jolenny1; Sotelo-Robledo, Roberto1; Juárez-Hernández, Fortunato1
ABSTRACT
Non-neoplastic mediastinal lesions of the mediastinum comprise a heterogeneous group of diseases that do not show a specific compartmental location; Radiologically, its characteristics can guide the diagnosis, the best follow-up method, as well as treatment and prognosis. Computed tomography (CT) and magnetic resonance imaging (MRI) features provide better patient selection for therapeutic interventions. The present review provides the radiological findings of this group of diseases.KEYWORDS
mediastinum, mediastinitis, lymphangiomatosis, pneumomediastinum, hemorrhage.Introduction
The group of diseases that make up the non-neoplastic lesions of the mediastinum have in common characteristics associated with increased thickness of the bands, displacement of lines or morphological modification of mediastinal recesses. Anatomical knowledge facilitates the decision making of the best diagnostic method that provides the most clinical information possible and the following therapeutic and/or prognostic procedure. The purist anatomical descriptions of the mediastinum have allowed, according to the structures included in it, to provide diagnostic possibilities based on compartments. However, nowadays the diagnostic tools have evolved technologically, allowing better image quality, better resolution of the anatomical structures and, therefore, descriptions with a broad semiological support that reduces the diagnostic options.
In the present review, we objectively provide the findings that best orient radiologically in the clinical-therapeutic approach, given that most non-neoplastic lesions are not limited to a mediastinal compartment.
Material and methods
We conducted a review of the literature. Only studies published in English were considered. The databases used included UpToDate, Medline and PubMed. The types of articles included in the search criteria were retrospective, prospective, randomized controlled trials, case report studies, original research, meta-analysis, abstracts, and previous related reviews. Search terms used to identify relevant articles during screening included: mediastinal cysts, mediastinal granulomatous disease, mediastinitis, lymphangiomatosis, pneumomediastinum, mediastinal hemorrhage, and diaphragmatic hernias.
Radiological features of non-
Mediastinal abnormalities are frequently detected on chest radiography by identification of abnormal mediastinal contour or displacement and/or increased thickness of mediastinal lines and bands. Approximately 10% of mediastinal contours are vascular and include anomalous vessels and aneurysms.1 Mediastinal abnormalities can be focal, unilateral or diffuse and bilateral; once the abnormality is identified, its location is corroborated in lateral projection and, according to this, its exact location is determined. Computed tomography (CT) and magnetic resonance imaging (MRI) allow the characterization of the lesions, evaluate their component in density or signal intensity, the effect on adjacent structures and their enhancement behavior.
Non-neoplastic pathology of the mediastinum can be classified according to its main etiology: 1) infectious type lesions: granulomatous lymphadenopathy and acute and chronic mediastinitis; 2) congenital type lesions: mediastinal lymphangiomatosis; 3) acquired non-infectious inflammatory lesions, post-birth: pneumomediastinum, mediastinal hemorrhage, diaphragmatic or hiatal hernia, esophageal dilatation and mediastinal lipomatosis; 4) cystic and pseudocystic lesions: congenital or acquired including bronchogenic cyst, esophageal duplication cyst, celomic cyst; intrathoracic pancreatic pseudocyst and hydatid cyst.
1. Infectious lesions
a. Granulomatous lymphadenopathy
When a mediastinal lymph node is visible in the chest X-ray, it modifies the mediastinal bands or lines depending on its origin and vascular drainage, they are generally larger than 10 mm, lose their kidney-shaped morphology and show a characteristic postcontrast enhancement. Most frequently, the paratracheal bands increase in thickness, modify the trajectory of the anterior pleural junction line, the azygoesophageal recess is convex and the contour of the aortopulmonary window is convex towards the parenchyma.
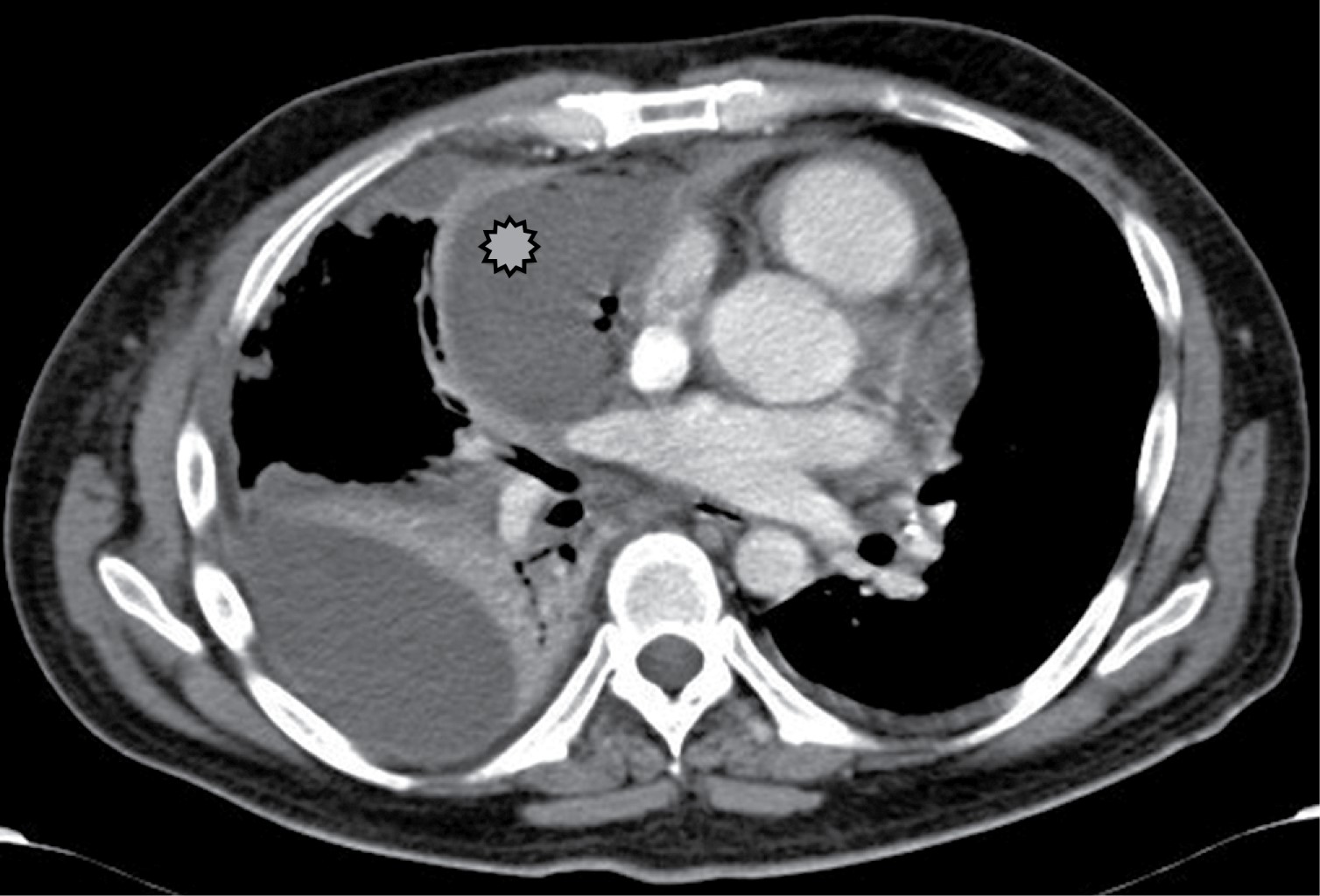
Non-metastatic or neoplastic nodal disease may have different radiologic findings depending on its etiology. When an organism can be identified, granulomatous lymphadenopathy in the mediastinum is usually caused by tuberculosis or endemic fungi such as histoplasmosis. Granulomas may result from a mass of lymph nodes that may compress adjacent structures, may induce an inflammatory reaction leading to sclerosing mediastinitis.
Mediastinal lymphadenopathy occurs in 7% of adults with tuberculosis, although lymph node enlargement is more common in AIDS. Lymphadenopathy may demonstrate low central attenuation; these areas of low density within the lymph nodes correspond to caseous necrosis (Figure 1).2 The right paratracheal area is most common, but may involve the anterior mediastinum or hilum. Lymph nodes may coalesce into poorly defined masses and adhere to adjacent vascular structures and mediastinal structures.
Between 5 to 13% of patients with histoplasmosis and nodal disease have esophageal involvement, particularly in the subcarinal region. Other less common causes include coccidioidomycosis, blastomycosis and cryptococcosis.2
Another effective imaging method for the evaluation of mediastinal and hilar lymph nodes is MRI, which has a lower spatial resolution than CT, but the use of paramagnetic contrast medium makes it possible to easily identify the lymph nodes. There are mediastinal levels that can be assessed more easily in MRI studies, the lymph nodes located in the aortopulmonary window are observed in coronal section, and the subcarinal lymph nodes are better seen in sagittal section.
b. Acute mediastinitis
It is an infection that is becoming less common since the advent of more effective antibiotics, it can be primary or secondary. Primary cases of mediastinitis are rare. Although the infection itself is self-limiting and resolves completely, it can also spread to the neck or broad ligament of the lung; 90% of mediastinitis is secondary to esophageal perforation or rupture.3
Descending necrotizing mediastinitis has an incidence of 1 to 2% and mortality between 30 to 67%. It is more frequent in men than in women, with a 6:1 ratio, the average age is 38 years, with a range of 28 to 30 years.4
The most frequently affected sites are superior (60%), anterior (60%), middle (20%) and posterior (20%) mediastinum. The most frequent finding on chest radiography is mediastinal widening (sensitivity of 77% and specificity of 66%) and pneumomediastinum (specificity of 100%).5
Chest CT is considered the modality of choice, it delimits the location and extent of the pathology, aiding clinical decision making when patients require immediate surgical intervention and cannot be managed conservatively by demonstrating pleural association and parenchymal disease.
The incidence of mediastinitis as a complication of cardiac operations is between 0.4 and 5% with a high mortality between 27 and 50% depending on the extent of the infectious process.3
Respiratory tract infections are rare causes of mediastinitis. However, oropharyngeal infections, such as tonsillitis, Ludwig's angina and retropharyngeal abscess, are cause for concern since they tend to spread along the fascial planes. These infections can cause necrotizing mediastinitis. The lateral parapharyngeal space is a transfer point for infections originating in the mandible, parotid glands, tonsils and cellulitis of the sublingual and submaxillary spaces.3,6
Tomographic findings include fluid collections, mediastinal gas, increased mediastinal fat attenuation, mediastinal widening, pleural effusion, pericardial effusion and presence of lymphadenopathy, in association with peristernal abnormalities such as soft tissue edema, sternal separation with marginal bone resorption, sclerosis and osteomyelitis (Figure 2). The first two findings are characterized in the literature as highly positive.7
Mediastinal collections of 20 HU or less is indicative of the presence of fluid contents; however, high densities suggest the presence of blood which does not exclude the presence of a concomitant infection.
The differential diagnosis of loculated fluid collections includes postsurgical seromas, which show no enhancement of their wall with contrast medium. However, liquid collections of mediastinitis may not show enhancement if they occur within the first postoperative week.
CT performed up to day 15 after surgery has a low specificity due to the short period of time elapsed. Therefore, pathologic findings are difficult to differentiate from those expected in the postoperative period in these types of procedures. Frequent postoperative symptoms such as fever or chest pain may justify a tomographic study for the investigation of mediastinitis.
c. Chronic mediastinitis (sclerosing or fibrosing mediastinitis)
Fibrosing mediastinitis is defined as diffuse fibrotic infiltration throughout the anterior, middle and posterior mediastinum. Organisms are difficult to demonstrate, and cultures are usually negative. The gap of differential diagnoses includes histoplasmosis, tuberculosis, coccidioidomycosis, actinomycosis, sarcoidosis, blastomycosis, syphilis, and various malignancies (carcinoma, sarcoma, mesothelioma, and lymphoma).
Fibrous tissue may proliferate within the mediastinum as a consequence of infection, usually histoplasmosis. Sclerosing mediastinitis may be related to systemic vasculitis and may have an immunologic pathogenesis. It has also been reported in autoimmune disease such as Behcet's, rheumatic fever, radiation therapy, trauma, and drugs such as methysergide. In addition, it may occur in association with other fibroinflammatory disorders such as retroperitoneal fibrosis, sclerosing cholangitis, Riedel's thyroiditis and orbital pseudotumor.
Neoplasms that frequently produce fibrosis and can then be included in the differential diagnoses include sclerosing non-Hodgkin's lymphoma and nodular sclerosing variant Hodgkin's disease.
Symptoms are caused by obstruction of the superior vena cava, esophagus, trachea, bronchus or pulmonary veins, pulmonary arterial hypertension due to direct compression of the pulmonary arteries, or secondary to pulmonary venous compression.8
Approximately 30% die from complications caused by obstruction and fibrosis.9 The worst prognosis is related to bilateral or carinal involvement. It is the most common cause of superior vena cava syndrome of benign origin. Central airway obstruction is by far the most common typical manifestation in patients with fibrosing mediastinitis presenting with cough and dyspnea. Causes of death are recurrent infection, hemoptysis, or cor pulmonale.
Fibrosing mediastinitis manifests as nonspecific mediastinal widening, with distortion and obliteration of recognizable mediastinal interfaces or lines. The middle mediastinum is most frequently affected, particularly the right subcarinal and paratracheal regions. Calcifications within the mediastinum or hilar are seen in 86% of patients.10
Central airway involvement may result in segmental or lobar atelectasis or recurrent pneumonia in the affected portions of the lung. The area of narrowing usually occurs at the level of the carina and in most cases in both bronchi.
Pulmonary arterial obstruction is typically unilateral and may result in an appreciable decrease in vessel size and number and localized regions of oligohememia in the affected segments. Venous obstruction manifests radiologically with findings of localized pulmonary venous hypertension, peribronchial cuffing, septal thickening or localized edema.
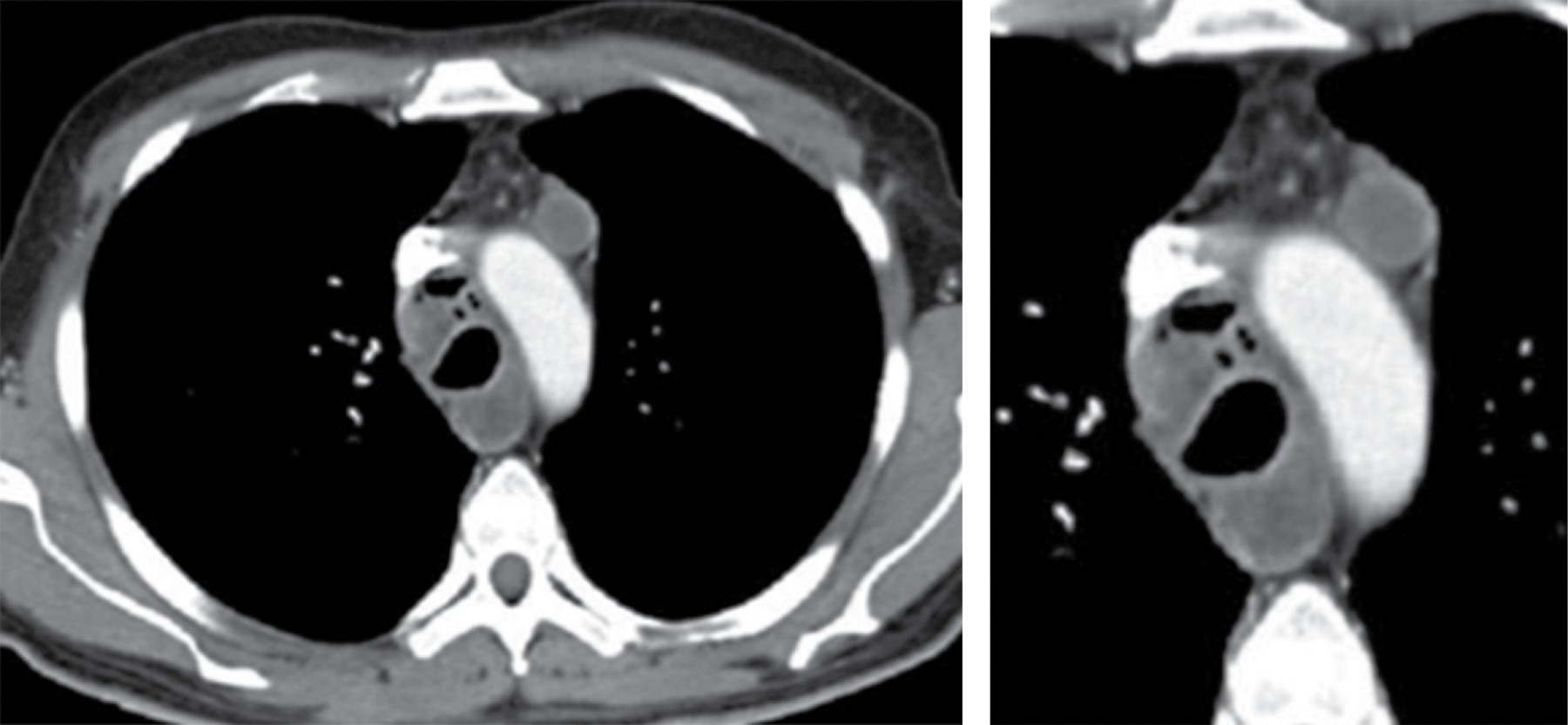
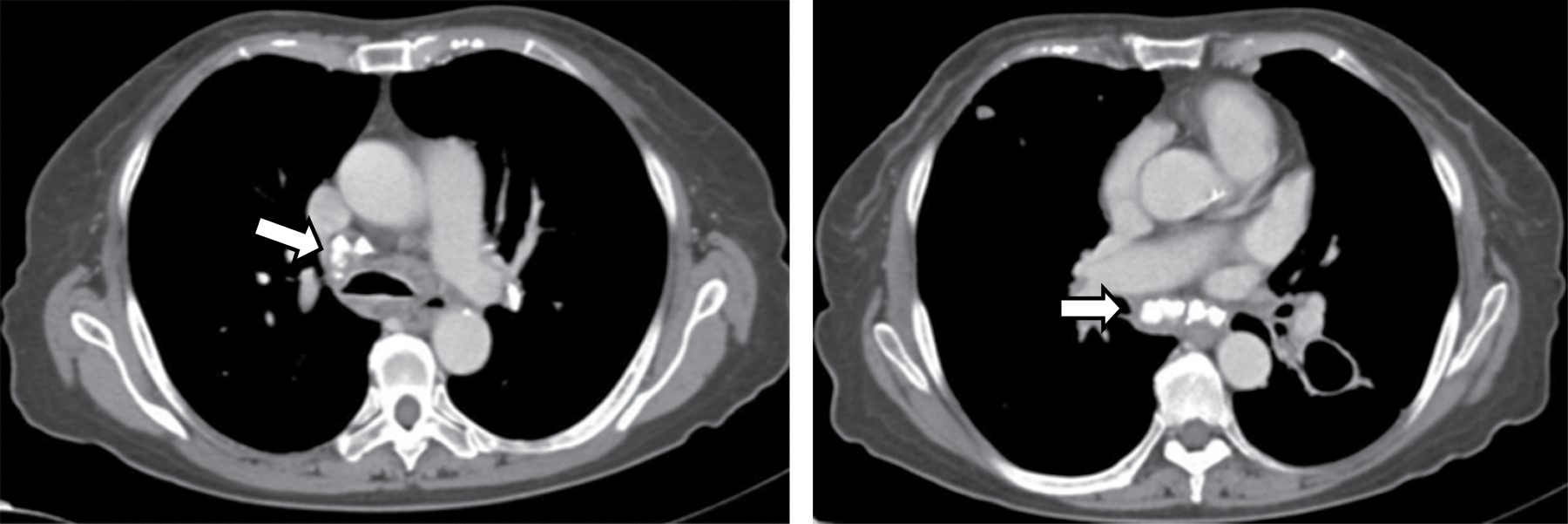
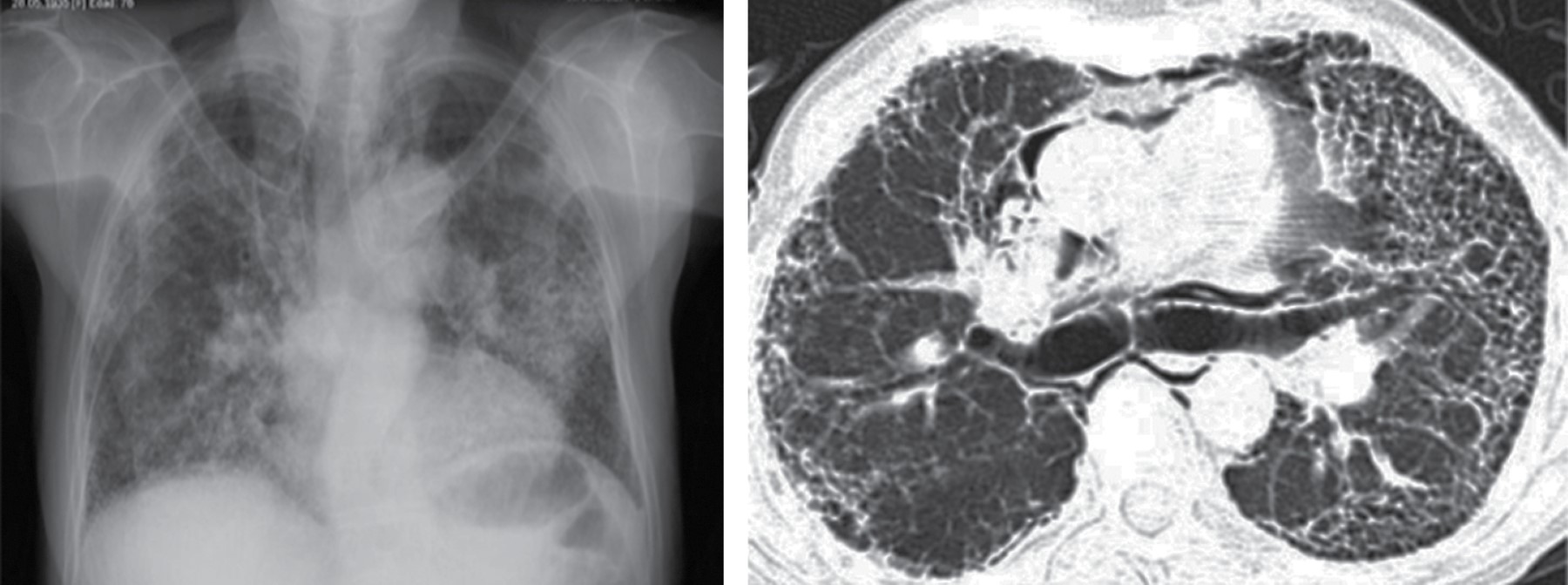
On CT, it typically manifests as soft tissue attenuation masses that obliterate mediastinal fat planes and encase or invade adjacent structures. Sherrik et al8 identified two patterns of tomographic invasion: a focal pattern and a diffuse pattern. The focal pattern (Figure 3) seen in 82% of cases, manifests as a mass with soft tissue attenuation that frequently calcifies (63%) and is usually located in the right paratracheal or subcarinal regions or in the hilum. The diffuse pattern (Figure 4), seen in 18% of cases as a non-calcified infiltrative mass affecting multiple mediastinal compartments. The diffuse pattern occurs in the setting of other idiopathic fibrosing disorders such as retroperitoneal fibrosis.
The degree of enhancement is variable and is useful to describe pigeonholing or obstruction of pulmonary arteries and veins. Two- or three-dimensional reconstructions can facilitate the surgical approach or local therapy of these lesions.
Venous obstruction frequently results in parenchymal abnormalities visible on CT, such as focal or diffuse regions of increased focal or diffuse parenchymal attenuation, ground-glass attenuation, and interlobular septal thickening.
CT also helps to evaluate the site, length and severity of airway stenosis. Esophageal invasion by fibrosing mediastinitis is best demonstrated by esophagogram. The junction of the upper and middle thirds of the esophagus is frequently affected, although extensive invasion can also occur. The affected segment of the esophagus is usually adjacent to invaded regions of the trachea or main bronchi. Typical findings include circumferential narrowing and large segmental strictures.
MRI shows features ranging from heterogeneous and infiltrative mass appearance in T1-weighted sequences, to areas of hyperintensity and hypointensity visualized in T2-weighted images. These T2 hyperintense areas are considered to be related to areas where there is more active inflammation, while those T2 hypointense areas represent areas of fibrosis and calcifications; with paramagnetic contrast medium, heterogeneous enhancement of the affected mediastinum can be observed.
2. Congenital lesions
a. Lymphangiomatosis
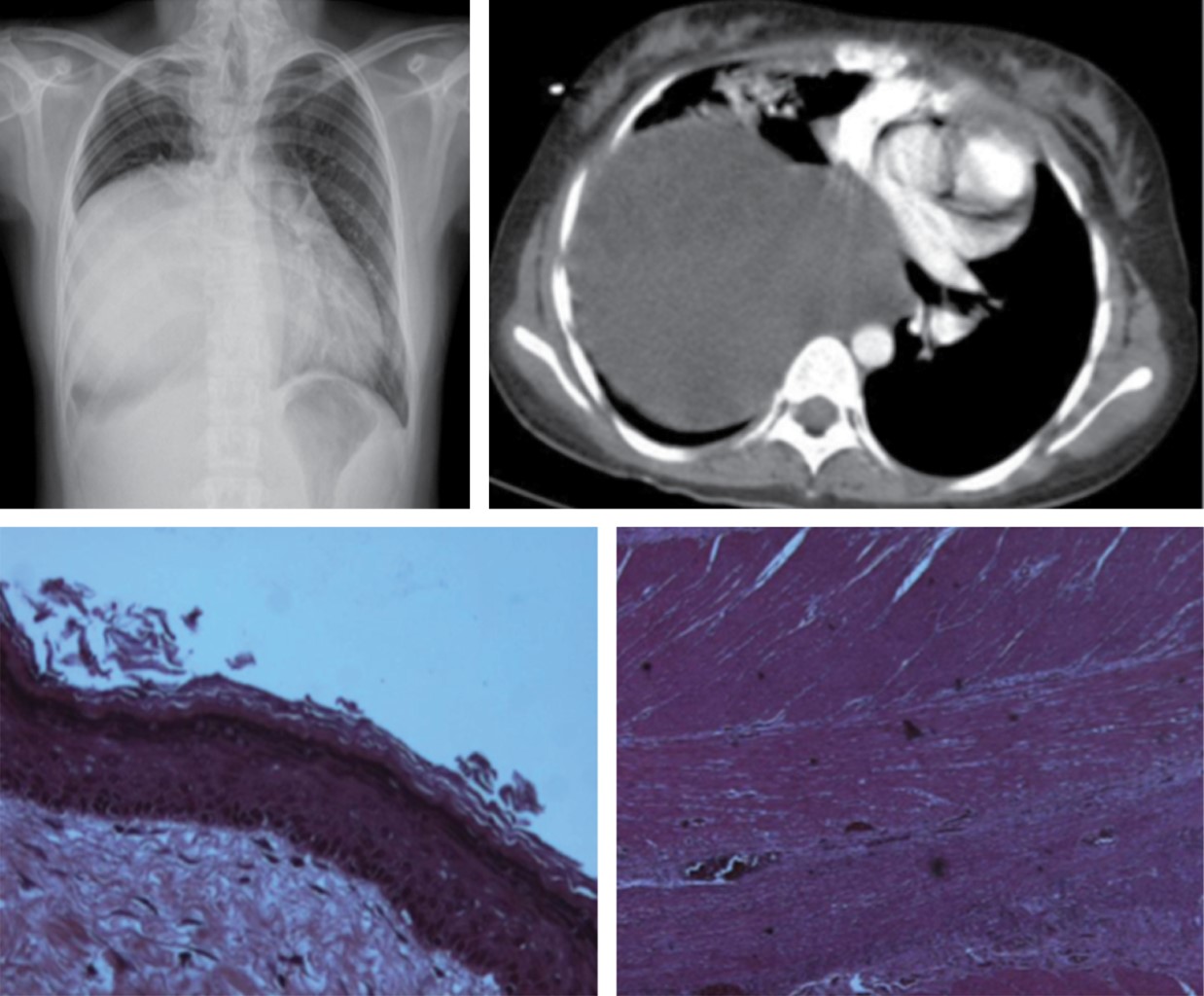
It is a diffuse proliferation of lymphatic vessels in multiple sites. It is more common in children. The mediastinum may be affected as well as the lung and pleura, presenting with chylothorax. In 60% of cases it is present at birth and in 90% the diagnosis is made during the first or second year of life.11
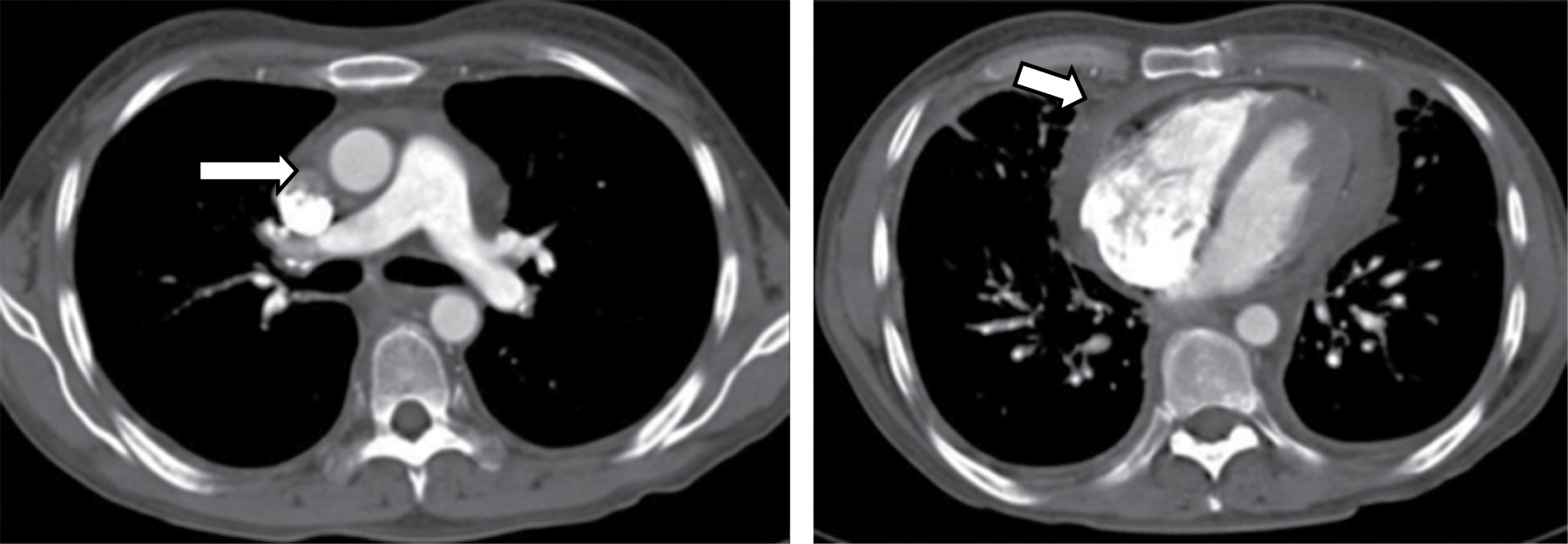
Radiological findings are: generalized mediastinal widening on CT with increased mediastinal fat attenuation (water-like). A focal mass is not visible. The involvement may be associated with interlobular septal thickening. Pleural thickening or pleural effusion is seen in almost all patients (Figure 5).
3. Non-
a. Pneumomediastinum
It was described in 1819 by Laennec. Macklin described the physiology of pneumomediastinum in 1939.12 Pneumomediastinum is manifested by lucent lines or gas bubbles that demarcate mediastinal structures, elevate the mediastinal pleura and frequently extend to the neck or chest wall.
The findings basically depend on the delimitation of the anatomical structures by air both radiologically and on CT. If there is sufficient air, the thymus may be elevated producing the "flying thymus" sign. Air anterior to the pericardium (pneumopericardium) is a frequent manifestation and requires a lateral chest view for diagnosis. Air surrounding the pulmonary artery or its main branches may result in a ring around it (artery sign). When there is air adjacent to the main branches of the aorta both sides of the vessels are demarcated; mediastinal air demarcates the medial side, and aerated lung margins the lateral side ("tubular artery sign").
Occasionally, air may be in front of a major bronchus, allowing clear distinction of the bronchial wall producing the "double bronchial wall contour sign". The continuous diaphragm is produced by air trapped posterior to the pericardium, which gives the appearance of a continuous collection of air on the anteroposterior radiograph. Mediastinal air may extend laterally between the parietal pleura and the diaphragm producing the extrapleural sign.
Air may also migrate into the mediastinum within the pulmonary ligament to rupture into the distal esophagus (Figure 6). Other names for pneumomediastinum include the "V of Naclerius" sign, in which gas delimits the margin of the descending aorta and extends laterally between the parietal pleura and the left medial hemidiaphragm. Although this finding was originally described in association with esophageal rupture, it is not specific for any other condition. A second "V-sign" is formed by gas delimiting the superior margin of the brachiocephalic veins at their confluence.
b. Mediastinal hemorrhage
Anterior mediastinal hematoma may occur as a sequela of rupture of a mediastinal vein (such as the internal mammary vein) after trauma or coronary artery catheterization. It may also be seen with aortic damage from blunt trauma, such as in a motor vehicle accident. In addition, anterior mediastinal hematoma has been reported to occur spontaneously in patients receiving hemodialysis.2
CT demonstrates a liquid collection, which has high attenuation and may or may not have air densities within it. Soft tissue edema in the chest wall, sternal or rib fracture and other findings are due to trauma and may be seen in these patients. Pseudoaneurysms can be carefully excluded in cases of anterior mediastinal hematoma; therefore, any evaluation of the chest in the trauma patient should be performed with bolus administration of intravenous contrast material.
When there is suspicion of mediastinal hemorrhage in MRI we can visualize a mass-like lesion with increased signal in FLAIR (fluid attenuated inversion recovery) enhanced images, which does not lose signal intensity in fat-suppressed sequences, and signal restriction in diffusion sequences, which expresses different stages of hemorrhage.
c. Hiatal hernia
The esophageal hiatus is formed by the decussation of muscle fibers originating from the diaphragm around the lower esophagus. Sliding hernias account for 90% of them, the remaining 10% are paraesophageal hernias.10
In a patient with a sliding hiatal hernia, the most common abnormality identified is dehiscence of the diaphragmatic crura and stretching of the esophageal brachial ligament. These findings manifest as widening of the esophageal hiatus identified when the medial margins of the diaphragmatic crura are not closely opposed. The current standard esophageal hiatus width measurement, defined as the distance between the medial margins of the crura, averages 10.7 mm with a maximum width of 15 mm.
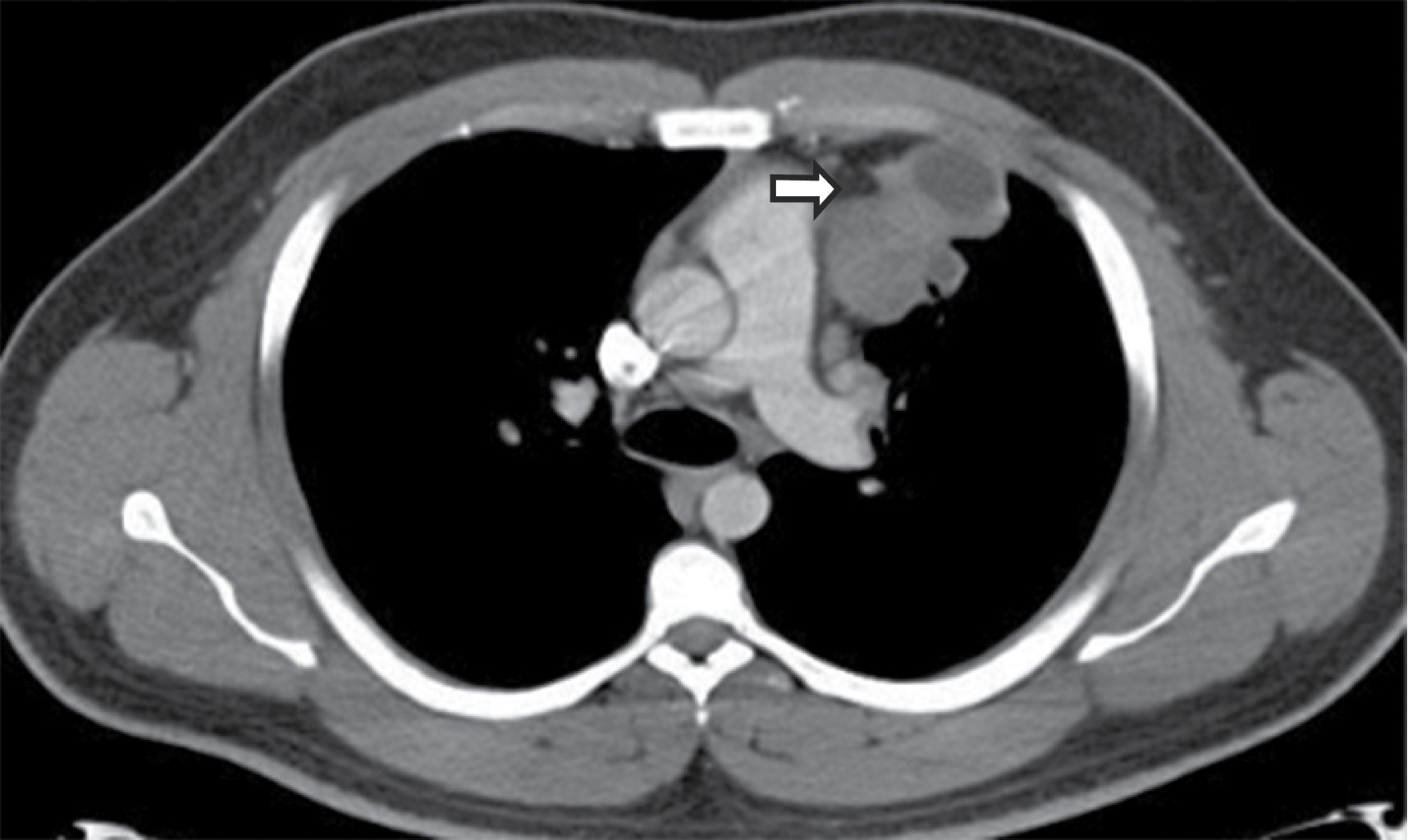
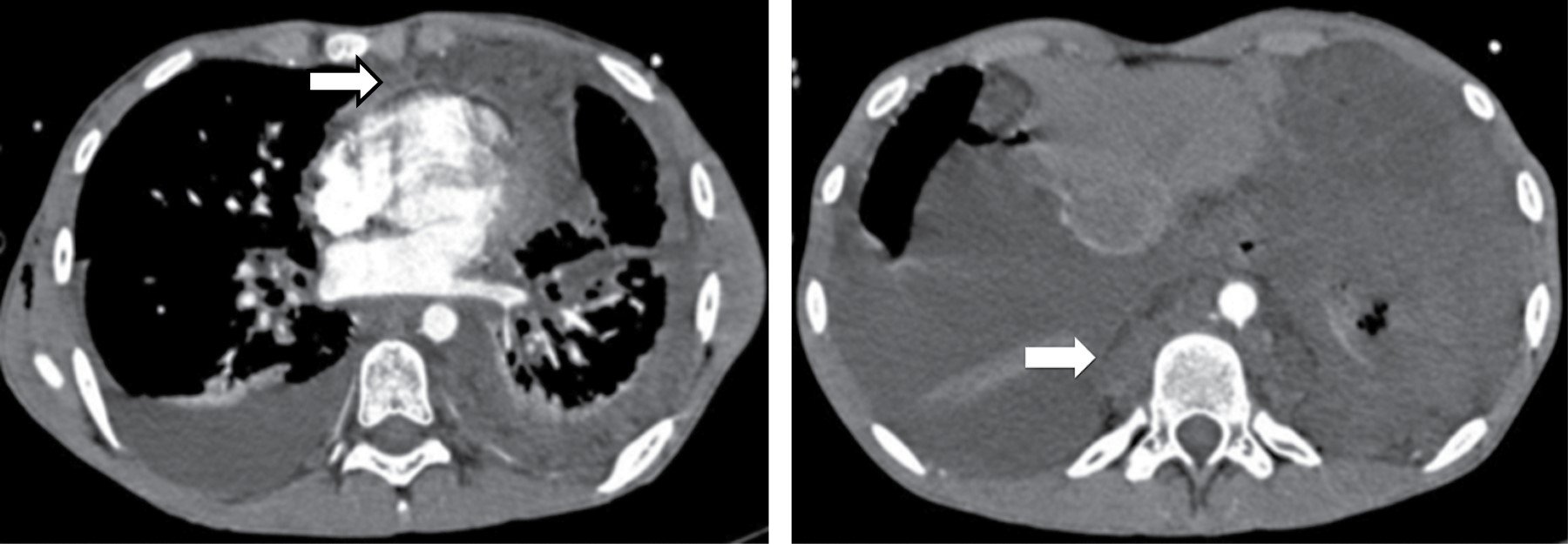
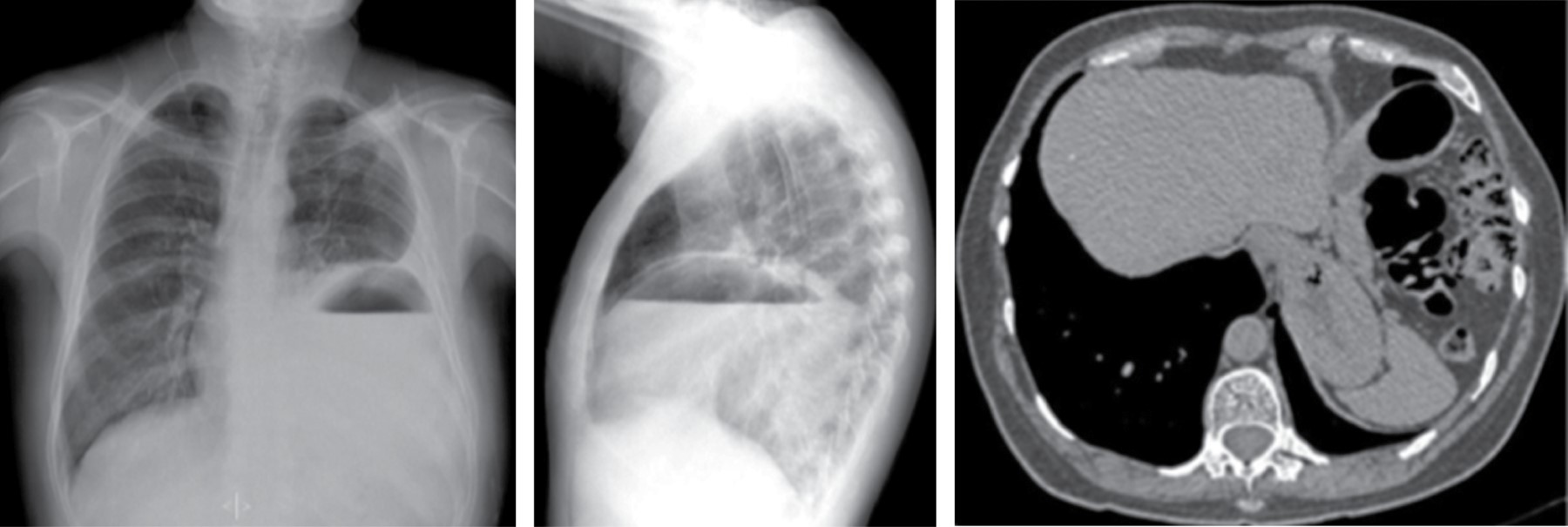
Sliding hiatal hernias are commonly associated with an apparent increase in mediastinal fat surrounding the distal esophagus secondary to omental herniation through the phrenic-esophageal ligament. Radiologically, the presence of a mediastinal mass with a hydroaerial level inside (Figure 7), allows the diagnosis of hiatal hernia, it can extend to the right, left or bilaterally, it displaces the azygoesophageal recess. Tomographic findings with a wide esophageal hiatus, direct visualization of abdominal contents, identification of the esophageal-gastric junction, may be associated with atelectasis, consolidation by microaspiration of gastric contents.
The main differential diagnosis is Bochdalek's hernia, which occurs through the remnant of the pleuroperitoneal canal.
d. Esophageal dilatation
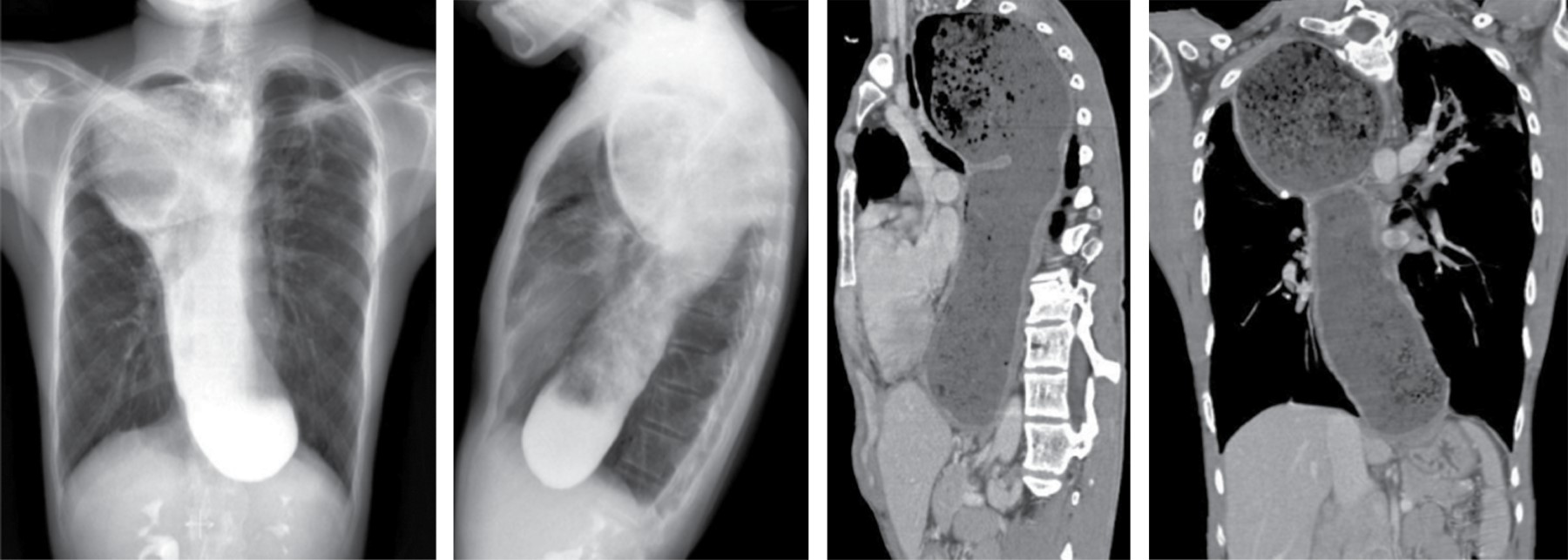
It may occur with esophagitis and strictures, esophageal carcinoma or other tumors, fibrosing mediastinitis, scleroderma, achalasia and leiomyomatosis. Marked dilatation may result in a mediastinal mass apparent on radiographs. In patients with known esophageal dilatation, CT may be used in identifying the mass (Figure 8).
Esophageal dilatation in achalasia and scleroderma is usually associated with normal wall thickness. An air-fluid level and retained food may be visible in patients with achalasia, stricture or carcinoma, but is less common with scleroderma. Esophageal dilatation is present in 80% of patients with scleroderma and is often asymptomatic.
Few descriptions exist on the application of MRI in the diagnosis of pathologies affecting the esophagus, but with the advent of real-time acquisition it has been possible to demonstrate some findings such as the classic bird's beak sign in achalasia, esophageal dilatation, the diameter of the esophageal sphincter and motility disorders by visualizing the dynamics of the transit of the bolus or saliva.
e. Mediastinal lipomatosis
It is a benign condition in which abundant amounts of histologically normal, unencapsulated fat accumulate in the mediastinum. It may be associated with Cushing's syndrome, steroid treatment or obesity. It has no symptoms. It is relatively common and is frequently detected in patients with chest CT.
Excess fat deposition is more prominent in the superior mediastinum resulting in smooth mediastinal widening as shown on chest radiograph and convex or bulging pleural surfaces on CT. Tracheal compression or displacement is absent. Less common is fat accumulation in the cardiophrenic angles and paraspinal regions.
In patients with lipomatosis, the fat should appear homogeneously low attenuating, sharply delimiting the mediastinal vessels and nodes.
Among the differential diagnoses are mediastinal fatty masses, as well as focal fatty lesions such as mediastinal fat necrosis, characterized by a nidus of juxtapericardial fat attenuation surrounded by inflammatory tissue, it is the analogue of epiploic appendagitis in the abdomen, and is accompanied by pericardial effusion and adjacent atelectasis. Mediastinal fat necrosis occurs frequently in men between 40 and 50 years of age, it is self-limited, the symptoms are similar to those perceived in pulmonary thromboembolism and acute myocardial infarction, they disappear in 48 to 72 hours.13 It is histologically normal fat, very easy to identify in MRI studies, increased signal intensity is observed in T1 and T2 sequences, with similarity to subcutaneous fat when compared.
4. Cystic and pseudocystic lesions of the mediastinum
Mediastinal cysts form a group of rare benign lesions of congenital and inflammatory nature, accounting for 20-32% of all primary mediastinal masses.14 They include different pathologic entities with overlapping clinical and radiologic features. They are seen in both adult and pediatric populations and their classification is based on the cause.
a. Bronchogenic cysts
Bronchogenic cysts constitute 50-60% of all mediastinal cystic lesions. These types of cysts are sometimes found together with other congenital lung malformations, such as pulmonary sequestration and lobar emphysema, thus calling them hybrid malformations.15
Cysts may have clear fluid, serous or mucoid material. They occur anywhere in the mediastinum, but frequently near the carina in the middle or posterior mediastinum. Less frequently they appear in the parenchyma, pleura or diaphragm. They are frequently connected by fibrous tissue to the trachea or bronchus. Cysts are spherical and usually unilocular, but may be multilocular. They have a thin wall with a smooth outer surface and a trabeculated inner lining.
On CT a bronchogenic cyst appears as a single smooth, round or elliptical mass with an imperceptible wall and uniform attenuation (Figure 9). The value, in Hounsfield units, may be greater than 100 HU if they have an elevated protein level and calcium oxalate in a mucoid cyst. Air within the cyst suggests infection and communication with the airway.
In MRI, in T2 bronchogenic cysts present increased signal due to their liquid content; in T1 enhanced images there is variation in the signal pattern, due to the presence of protein, hemorrhagic or mucous content inside the cyst, even up to the presence of liquid-liquid level.
b. Enteric duplication cyst
Mediastinal enteric cysts, also called enteric duplication cysts, are esophageal or gastroenteric cysts. They are mainly diagnosed in children under 15 years of age. A male predominance has been described. Patients have respiratory symptoms, other symptoms present include dysphagia, cough and vomiting. Cysts covered by gastric epithelium may ulcerate and perforate. These cysts are differentiated from bronchogenic cysts by location, absence of cartilage and the presence of muscularis propria. The nature of the cyst sometimes cannot be determined due to the absence of distinctive features.
They are frequently reported in association with malformations of the thoracic and cervical vertebrae. They are visually identical to bronchogenic cysts, except that the wall of the lesion is thick and in contact with the esophagus (Figure 10), they are located in the posterior mediastinum near the esophagus, typically in the retrocardiac position. On T1-weighted MRI images they are hypointense, while on T2-weighted images they are hyperintense and, occasionally, liquid-liquid levels are identified.
c. Coelomic cysts
Pericardial or coelomic cysts originate in the embryologic development of the pleuropericardial membranes, some are attached by a pedicle. They are usually reported in adults, but can also appear in children, many patients are asymptomatic. The cysts have fibrovascular tissue with a smooth, thin wall, contain fluid, are spherical and unilocular. The cyst contents are clear watery or straw colored. They are bounded by a plate or sheet of cuboidal mesothelial cells supported by loose connective tissue. Coelomic cysts can be treated surgically or by percutaneous aspiration of the contents.15
They usually originate in the cardiophrenic angle, they are more frequent in the right angle than the left. Some are in the superior mediastinum and connected to the pericardium. On CT, they can be seen as well-demarcated, unilocular, oval or round or triangular masses and can be 30 cm in diameter, the attenuation is similar to that of water. In MRI they present similar findings to the other cystic lesions of the mediastinum.
d. Hydatid cyst
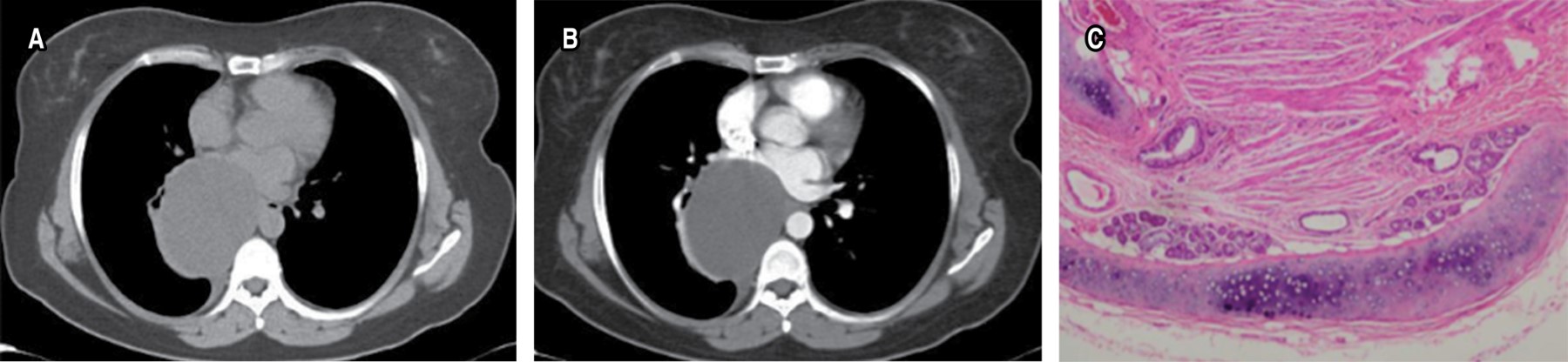
They are acquired cystic lesions, representing 0.1% of all mediastinal cysts. They are composed of a fibrous capsule: pericyst. The true cyst has a thin wall composed of two adherent layers, the laminated endocyst and the delicate tapetum endocyst, from which the daughter cysts hang. They have a predilection for the anterior mediastinum and appear as cystic lesions with daughter cysts (Figure 11).
In MRI the findings are very characteristic, the pericyst is hypointense in T1 due to its fibrous component. The mother or true cyst is of intermediate signal intensity on T1 and the daughter cyst will have lower signal intensity on T1 than the matrix of the mother cyst. On T2-weighted images, the pericyst will remain hypointense, and on T2 the mother and daughter cysts will have the same high signal intensity.
e. Lymphatic cysts
Cystic lymphangioma or cystic hygroma is a cystic lesion of lymphatic vessels. Thoracic duct cysts can have several mediastinal locations: below the azygos vein in the posterior mediastinum, above the aortic arch, at the level of the hilum, in the epiphrenic area and above the heart. They can measure 15 cm in diameter or more. The microscopic appearance is characteristic of fibrous connective tissue with endothelial cells. On CT, the lymphangioma typically appears as a multiloculated, smooth-margined mass with homogeneous water-like attenuation. On T1 and T2 MRI, adequate characterization of serpentiginous or vascular shaped septa are identified within.
f. Pancreatic pseudocyst
Alcoholism is a common factor in adults and trauma in children. In many cases, the pseudocyst extends from the pancreas to the posterior mediastinum through the esophageal hiatus. Less commonly, it can penetrate through the aortic hiatus, Morgagni's foramen or a diaphragmatic erosion.
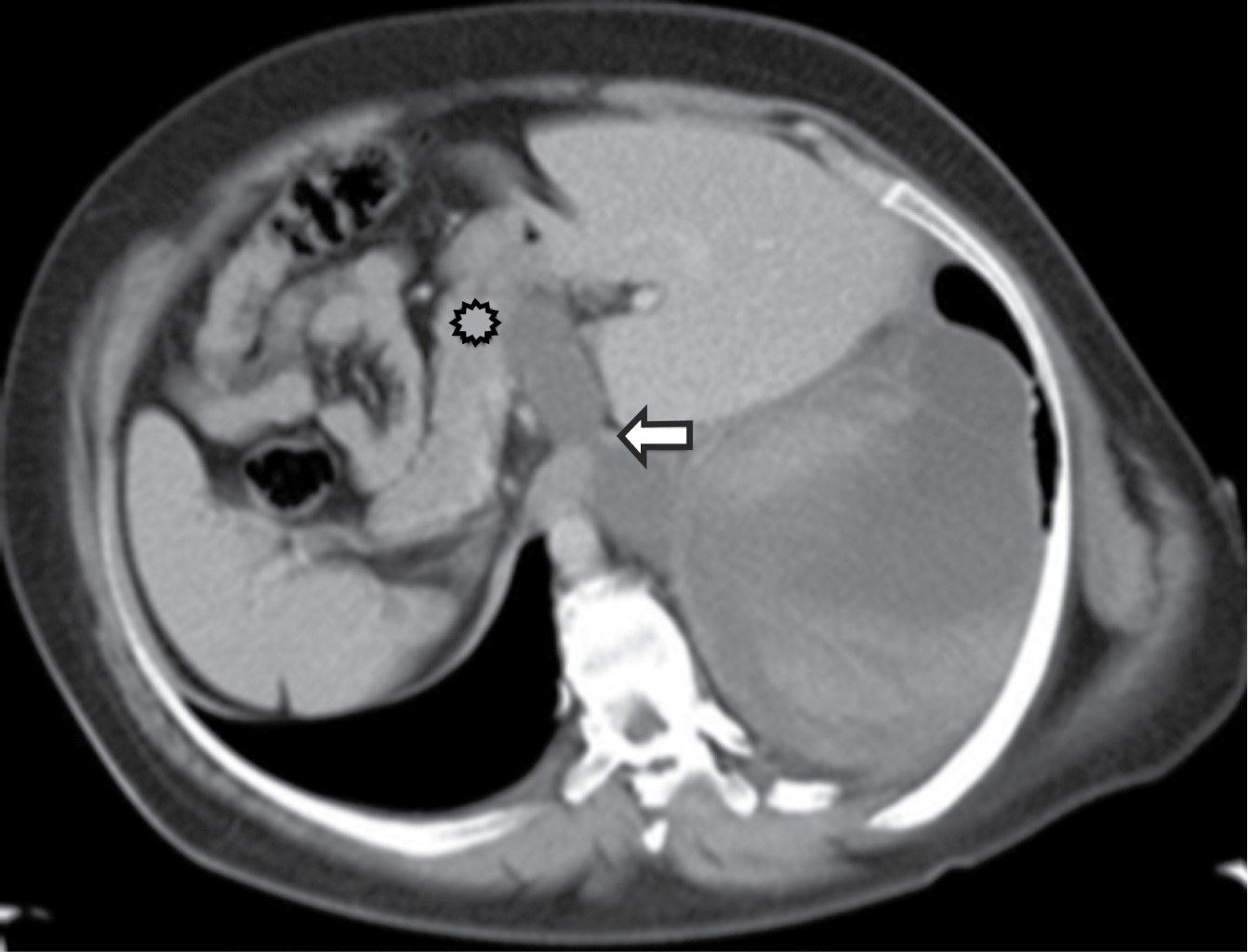
It represents an encapsulated collection of pancreatic secretions, blood and necrotic material. It always occurs in the lower posterior mediastinum, gaining access from the thorax via the esophageal or aortic hiatus. Tomography shows a thin-walled, low-attenuation cyst in the posterior mediastinum or adjacent to the thoracic cavity associated with compression or displacement of the esophagus, may be hyperattenuating depending on whether there is hemorrhage or infection (Figure 12).
Conclusion
The theoretical boundaries of mediastinal compartments are not so clear, and identification of the close anatomic relationships of a mass is often instructive.
In most cases, the initial use of chest radiography instructs the next diagnostic method upon suspicion of a visible mediastinal abnormality; however, tomography is a tool that in most cases is diagnostic in the context of a non-neoplastic lesion of the mediastinum, in short and long-term follow-up for clinical management and treatment.
There are several clues in the differential diagnosis, most of the time based on previous clinical knowledge.
Acknowledgments
Department of Anatomic Pathology, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City.
AFILIACIONES
1Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City.Conflict of interests: the authors declare that they have no conflict of interests.
REFERENCES
Gürsoy S, Ozturk A, Ucvet A, Erbaycu AE. Lesiones quísticas primarias y benignas del mediastino en el adulto: espectro clínico y tratamiento quirúrgico. Arch Bronconeumol [Internet]. 2009;45(8):371-375. Disponible en: https://www.archbronconeumol.org/en-benign-primary-cystic-lesions-mediastinum-articulo-S1579212909729349