Neutrophils as defense cells? Immunobiology and pathophysiology in human respiratory infectious diseases
Rojas-Valles, Edwin U1,2,4; Magaña-González, Carlos Alberto3,4; Herrera-Barrios, María Teresa2
Rojas-Valles, Edwin U1,2,4; Magaña-González, Carlos Alberto3,4; Herrera-Barrios, María Teresa2
ABSTRACT
The immune system protects us from infections and the entry of any pathogen activates innate immunity. Neutrophils are part of this type of response and are the most abundant in the blood with a short half-life that increases when they are activated. They are generated in the bone marrow during granulopoiesis and their release into the blood depends on the binding of CXCR4-CXCL12. They are the first cells to reach the site of infection or inflammation, and their bactericidal mechanisms are phagocytosis, degranulation, production of ROS, NET, cytokines, and chemokines. During infections, they carry out phagocytosis characterized by direct phagosome-granule fusion, and the pathogens are killed by the action of toxic granule proteins and oxidant molecules (ROS and hypochlorous acid). Pathogens or cytokines promote degranulation which, together with the production of ROS and hypochlorous acid, act on proteins, DNA, and bacterial membranes favoring their elimination. Neutrophils produce NET to trap pathogens and prevent their spread, and they are also a source of cytokines and chemokines, which is why they participate in the regulation of the immune response. In human infectious diseases, their participation can help, or contribute to a poor prognosis, causing tissue damage. This review aims to know the generalities of neutrophils and their participation in human respiratory diseases such as COVID-19 and influenza, tuberculosis, and histoplasmosis.KEYWORDS
neutrophils, COVID-19, influenza, tuberculosis, histoplasmosis.Abbreviations:
Introduction
The immune system includes cells involved in innate immunity or acquired immunity to maintain body homeostasis. The entry of any pathogen triggers an innate response that is quick to eliminate the pathogen and prevent disease. This is mediated by the recognition of pathogen-associated molecular patterns and cellular damage-associated molecular patterns without generating immunological memory. Neutrophils are part of this response and their bactericidal mechanisms are phagocytosis, degranulation, production of reactive oxygen species (ROS), neutrophil extracellular traps (NET), cytokines and chemokines.
1. Neutrophils
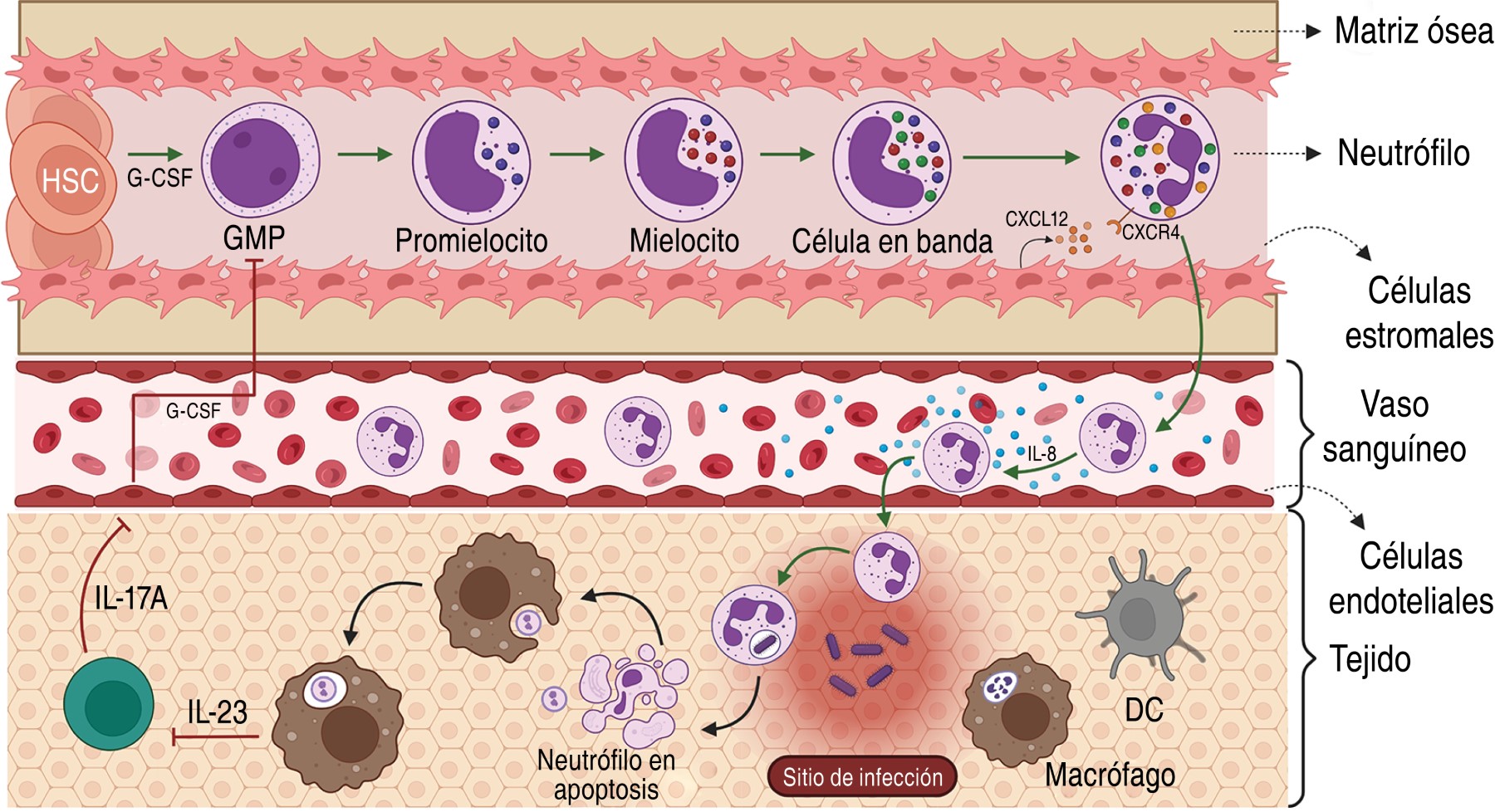
1.1 Origin and characteristics: they are generated in the bone marrow by granulopoiesis from a myeloid precursor and it has been estimated that a healthy adult produces 1-2 × 1011. Hematopoietic stem cells are located in the spaces created by osteoblasts and endothelial cells characterized by low blood flow and lower oxygen tension, while the more mature and cell-dividing cells are near the abluminal side of the sinusoids, a special vascular structure of the bone marrow.1 After maturation, neutrophils are released into the blood and this process depends on the interaction of their chemokine receptor CXCR4 and the chemokine CXCL12 produced by stromal cells in the bone marrow.2
Their homeostasis is regulated by phagocytosis of apoptotic neutrophils by macrophages and dendritic cells in tissues, reducing their proliferation in an IL-23/IL-17A/G-CSF axis-dependent manner.3 Phagocytosis decreases the production of interleukin 23 (IL-23), causing decreased production of IL-17A by neutrophil regulatory T lymphocytes or Th17 (Tn/Th17), which are located in mesenteric lymphoid nodules.4 Consequently, low levels of IL-17A decrease the production of granulocyte-colony stimulated factor (G-CSF) by fibroblasts and endothelial cells reducing the production of mature neutrophils. On the other hand, inflammation or infection causes an increase in G-CSF favoring granulopoiesis, production and recruitment of neutrophils.2,5 In addition, neutrophil homestoasis involves their cell death by necrosis, necroptosis, NET and pyroptosis (Figure 1).6,7
They constitute 50-70% of circulating leukocytes, with a diameter of 7-10 µm, a segmented nucleus, high granule content and the presence of secretory vesicles in their cytoplasm.2,5 Its half-life is eight to 20 hours without stimulus, although after its migration to tissues it lasts 1-4 days.6
They recognize pathogens through their membrane receptors such as: scavenger receptors, mannose receptors, Dectin 1, CD14, FcγR, CiqR, CR1, CR3, colectins, Toll-like receptors, NOD-like receptors.7
Their cytoplasmic granules are classified into: Primary/azurophil granules, containing myeloperoxidase (MPO), serine proteases, neutrophil elastase (NE), proteinase 3, cathepsin G, azurocidin, α-defensins (HNP-1, HNP-2, and HNP-3), serprocidins, and Bactericidal-permeability-increasing protein (BPi). Secondary/specific granules, containing matrix metalloproteinase 8 (MMP8), lactoferrin, LL-37, lipocalin 2, haptoglobin, Pentraxin 3 and olfactomedin 4. Tertiary granules/gelatinase contain gelatinase B, MMP8, MMP9, Arginase-1, LL-37 and lysozyme, and secretory vesicles containing albumin, cytokines, membrane receptors (CR1, CR3, C1qR, FcγR, CD14, FPR1), cell adhesion molecules (CD11b/CD18, CD67) and part of the nicotinamide adenine dinucleotide phosphate oxidase (NADPH) complex. The granule proteins are acquired during granulopoiesis.2,5,8,9
2. Bactericidal mechanisms
2.1 Phagocytosis: is a process of ingestion and elimination of particles or pathogens that enter the organism larger than 0.5 µm, including apoptotic bodies.10,11 Neutrophils, macrophages, monocytes and dendritic cells are classified as professional phagocytes, as they perform this task with great efficiency,10 while fibroblasts, epithelial and endothelial cells are considered non-professional phagocytes in charge of eliminating dead cells to maintain homeostasis.10,11
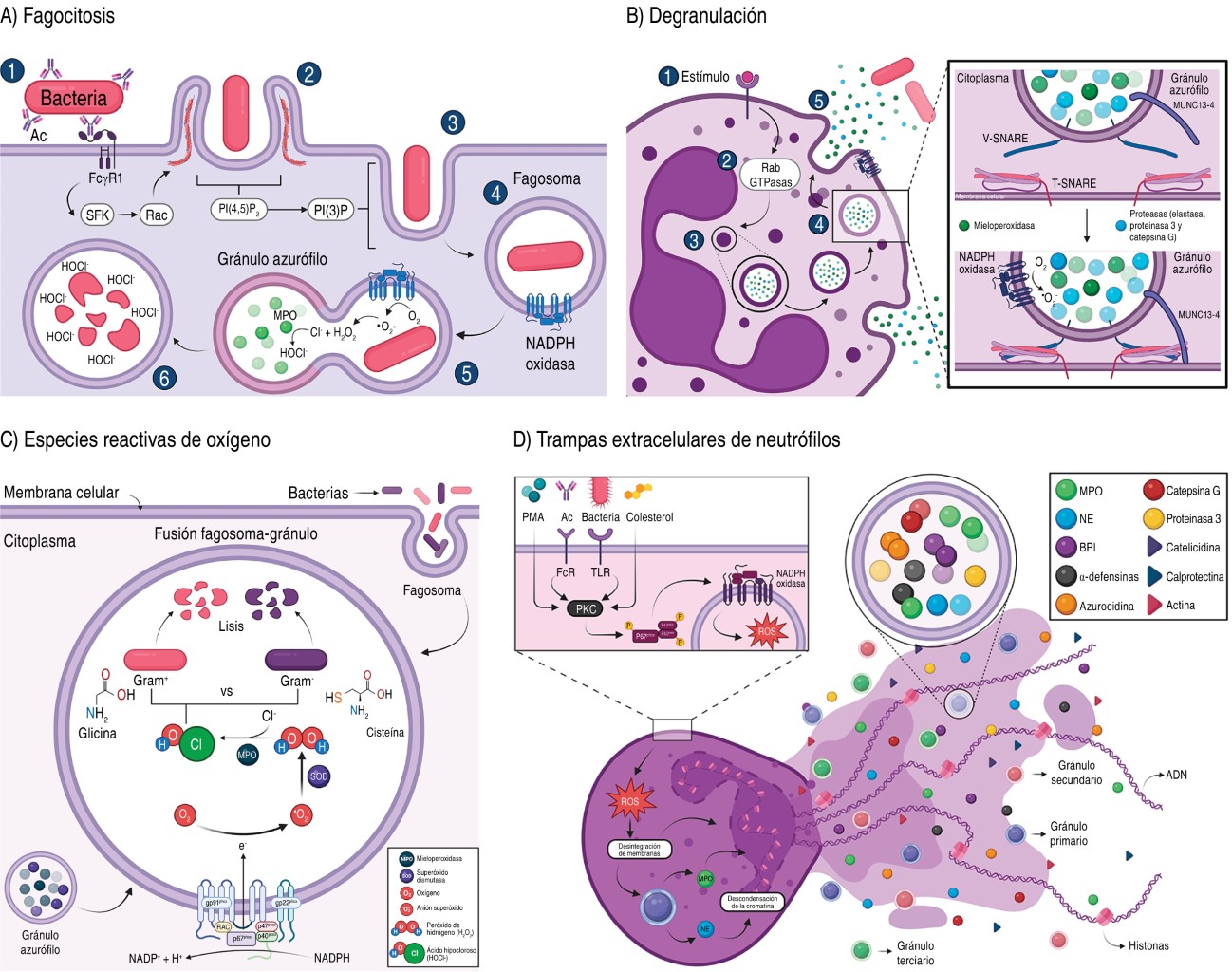
It starts with ligand binding to phagocytic receptors, which are divided into opsonic receptors such as: IgG crystallizable fragment receptors (FcγR) and complement receptors (CiqR, CR1, CR3); and non-opsonic receptors such as: mannose receptors, Dectin 1, CD14, collectins, TLRs, C-type lectin and scavenger receptors.10,12 Compared to macrophages, phagocytosis by neutrophils is rapid as the phagosome directly fuses with the cytoplasmic granules in less than 60 seconds, allowing rapid clearance of pathogens (Figure 2A).13,14
Binding of the pathogen or particle causes a rapid oxidative and non-oxidative response by the assembly of the NADPH oxidase complex on the phagosome membrane.9
2.2 Degranulation: these cells also combat extracellular pathogens by releasing their cytoplasmic granules. Two types of signals are required: a) β2 integrin-dependent; and b) activation of receptors such as Mac1/CR3, FcγR and G Protein-Coupled Receptors (GPCR). This process involves the Rab and Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins involved in the control of vesicular trafficking (Figure 2B).
Degranulation of secretory vesicles and tertiary granules is rapid; however, primary granules require neutrophils preactivated with proinflammatory cytokines, chemokines or microbial components. Neutrophils prevent excessive degranulation of tertiary granules by increasing ROS production, as the dysregulated process can cause tissue damage.2
Phagocytosis and degranulation cause the assembly of the NADPH oxidase complex at the membrane, causing the production of ROS.9,15
2.3 Production of reactive oxygen species (ROS): the interaction of neutrophils with the pathogen triggers the oxidative burst involving the NADPH oxidase-NOX2 enzyme system, which assembles on the cell membrane and generates two •O2- molecules and the enzyme superoxide dismutase generates H2O2 that acts as an antimicrobial by reacting with the thiol groups of enzymes, proteins, DNA and bacterial membranes.11,16-18
In addition, MPO utilizes H2O2 and catalyzes the reaction with chloride ions, forming a hypochlorous acid (HOCl-) that is highly reactive with thiol groups and methionine residues (Figure 2C).11,19
It is important to mention that some pathogens have generated a defense against ROS, but neutrophils possess other alternative bactericidal mechanisms.20
2.4 Production of neutrophil extracellular traps (NETs): neutrophils are killed by NETosis where NETs are generated, which are extracellular fibers composed of DNA, cytosolic proteins and antimicrobial granules that trap, neutralize and eliminate pathogens. This process begins with the loss of the lobular shape of the nucleus and disassembly of the nuclear membrane, loss of the permeability of the granular membranes, inactivation of histones by the action of NE that degrades the central histone H1 causing the decondensation of chromatin, causing the mixing of chromatin in the cytosol with the cytosolic and granular components, as well as the loss of the permeability of the cell membrane that allows the release of NETs into the extracellular space (Figure 2D).15,21,22
It has been reported that the proteins identified in NETs may vary depending on the stimulus, since with the stimulus Phytohemagglutinin (PMA, Phorbol-Myristate-Acetate) 24 proteins were identified, and with Pseudomonas aeruginosa 80 proteins were identified;15,21 although histones, NE, MPO, calprotectin, cathelicidins, α-defensins and actin are always found.21
NETs are formed by two pathways: a) NADPH oxidase-dependent (lytic, most studied) which is activated by antibodies, microorganisms, cholesterol and mitogens (PMA, concanavalin A).23,24 The stimuli activate protein kinase C (PKC), which activates the assembly of NADPH oxidase to the membrane, initiating the production of ROS, which disintegrate the membranes of the nucleus and granules, allowing NE and MPO to interact with histones to facilitate chromatin decondensation.23,24
On the other hand, b) in NADPH oxidase-independent (non-lytic, less studied) the nucleus condenses and the nuclear membranes separate, forming vesicles with DNA that are expelled into the extracellular medium, releasing chromatin.25 This mechanism prevents the spread of pathogens, although it also has direct bactericidal properties. For example, NE acts on outer membrane proteins and virulence factors of enterobacteria.15 The presence of NET is implicated in inflammatory and autoimmune disorders, such as acute respiratory distress syndrome, thrombosis in COVID-19, and in rheumatoid arthritis.22
2.5 Cytokine and chemokine production: neutrophils are a source of cytokines and chemokines to interact with T lymphocytes, B lymphocytes, macrophages and dendritic cells; participating in the regulation of innate and acquired immunity.26 They produce pro- and anti-inflammatory, immunoregulatory cytokines, G-CSF and important CXC-type chemokines in cell migration into the tissue and vice versa (Table 1).27-34
3. Neutrophils in infectious diseases
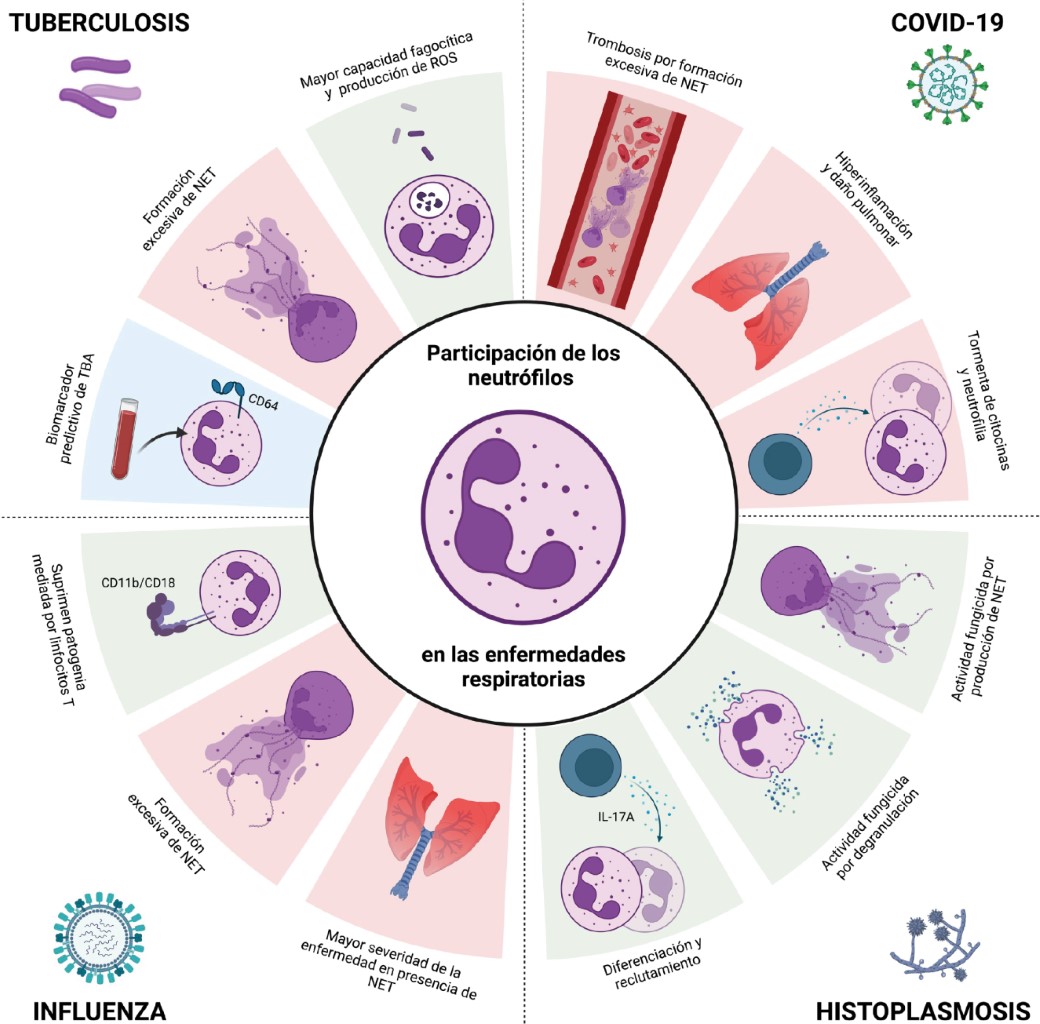
3.1 COVID-19: in December 2019, a new form of coronavirus called SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), a single-stranded, positive-sense enveloped RNA virus belonging to the β-coronaviruses, spread in Wuhan, China. The World Health Organization (WHO) declared the pandemic on March 11, 2020.35 Until November 16, 2023, there were 772,011,164 confirmed cases and 6,979,786 deaths worldwide.36 In patients with COVID-19, the presence of NE, MPO, citrullinated histone 3 (cit-H3), NET and platelets have been shown to be associated with vascular occlusion, necroinflammation and oxidative stress.37
In the severe form of COVID-19, inflammation and "cytokine storm" (IL-1β, IL-2, IL-6, IL-7, IL-8, IL-17, TNF, IFN-γ, IP-10, GM-CSF, MCP-1 and IL-10) have been described leading to the development of acute respiratory distress syndrome.38,39 Neutrophil recruitments to the site of infection and the formation of NETs have been described as contributing to thrombus formation and respiratory distress (Figure 3).39
The neutrophil-lymphocyte ratio (NLR) based on the number of neutrophils and lymphocytes in the blood together with the neutrophil-albumin ratio (NAR) are considered as biomarkers of infection and systemic inflammation. NLR values are useful for prognosis, as values less than 3 indicate mild systemic inflammation, 3 to 5 moderate inflammation, and greater than 5 are indicative of severe inflammation,40 acute respiratory failure syndrome is the primary cause of death in COVID-19 patients. Together, neutrophilia, NLR and NAR in the early stages of infection correlate with the severity of infection.41
In the severe form of COVID-19 there is neutrophilia in blood and lung tissue with increased IL-1β, IL-6 and D-dimer; while NETs have the potential to propagate inflammation, microvascular thrombosis and cytokine storm in the lungs.42,43
In vascular occlusion NLR, MCP-3 (monocyte chemotactic protein-3) and IL-8 promote neutrophilia in patients with mild and severe COVID-19, forming aggregates of neutrophils and thrombocytes that target mainly the pulmonary vessels,44 platelet-fibrin complexes target small pulmonary arteries and thrombi target pulmonary capillaries.42 Neutrophils and NETs favor necroinflammation,45 by infiltration of NET aggregates that form thrombi in pulmonary vessels, inducing vasculitis and finally necrosis that favors cytokine storm, causing further inflammation.46
Among the critical complications of COVID-19 is thrombosis which has been associated with elevated levels of free DNA, cit-H3, MPO-DNA complex and NET identified in arteriolar microthrombi. Released DNA, NET, MPO and cathepsin G have cytotoxic effects on pulmonary epithelium and endothelial cells (Table 2).
Potential markers of NET associated with symptoms have been described, for example: elevated levels of DNA, citH3, NE and the MPO-DNA complex are associated with admission to intensive care, mechanical ventilation and short-term mortality. The MPO-DNA complex has been associated with sequential organ failure, NE and Histone-DNA associated with lung damage, renal failure, body temperature and MPO associated with days with severe hypoxia.41 The release of NETs and ROS causes imbalance between ROS production and antioxidant mechanisms, increasing tissue injury.47
3.2 Influenza: caused by viruses of the genus Influenzavirus, belonging to the Orthomyxoviridae family, a negative-sense monocaterial RNA virus, which are transmitted by aerosols affecting cells of the respiratory tract and type II pneumocytes.48,49 Annual epidemics result in 3 to 5 million severe cases and 290,000 to 650,000 deaths.50
Influenza A, B, and C viruses affect humans, but only A and B are of medical importance.49,51 influenza type A virus (IAV) is the most common virus and has several subtypes, which are classified according to their antigenic variation in their surface proteins: hemagglutinin and neuraminidase.49
It causes seasonal epidemics and manifests as an acute illness with mild to severe symptoms; however, it can become complicated leading to hospitalization or death.48,49 Complications affect at-risk groups (children or older adults) and those with comorbidities (chronic heart or lung disease, diabetes mellitus and immunosuppression).48
Neutrophils contribute to disease control, and in IAV infection in the murine model, neutrophils and their adhesion molecules (CD11b/CD18) are important in limiting T cell-mediated pathology.52 However, although the virus induces an innate immune response characterized by neutrophil infiltrate in the lungs and mechanisms that promote resolution of the infection, they also contribute to the pathogenesis of severe disease.53 Despite tissue damage, lack of neutrophils is associated with increased lung damage.53,54
In patients with influenza, neutrophils and NETs are associated with greater severity.55 In mice co-infected with IAV and Staphylococcus aureus there is excessive recruitment of neutrophils to the lungs and NETs, which contribute to severe pulmonary inflammation.56 Similarly, patients with severe IAV H1N1 and H7N9 infection have elevated levels of NETs associated with disease severity and poor prognosis.57
3.3 Tuberculosis: WHO reported 10.6 million of new cases with tuberculosis and 1.6 million deaths in 2021.58 M. tuberculosis causes tuberculosis (TB) and is transmitted by aerosols. It causes asymptomatic infection (latent TB) in 90-95% and active TB in 5-10% of infected persons, causing mainly pulmonary TB (PTB).58-61
Protection depends on the innate and acquired immunity generated. Neutrophils participate in the response to M. tuberculosis during early infection, carrying out phagocytosis, production of ROS, cytokines and chemokines.62 However, although they participate in the immune response, they are not very crucial in the resolution of the disease, probably because they are cells with a short half-life.63 They participate in macrophage recruitment and promote inflammation and granuloma formation to contain infection.60,62-64
They contribute to TB resistance by producing antimicrobial peptides (LL-37 and lipocalin 2) that participate in the elimination of mycobacteria.65,66 Macrophages phagocytose NETs generated by infection with M. tuberculosis and produce IL-1β, IL-6, TNF-α and IL-10, evidencing their participation in the modulation of the immune response.67 NETs are in the plasma of TB patients and the increase correlates with the severity of the disease.68,69
In the search for biomarkers, the FcγR1 receptor (CD64) has been found to be increased on neutrophils and monocytes of patients with active TB, and may be a predictive biomarker of disease.59,70
3.4 Pulmonary histoplasmosis: caused by the inhalation of Histoplasma capsulatum microconidia or mycelial fragments, it is an endemic mycosis that affects more than 60 countries.71 With high incidence in North America and in tropical areas of Latin America with temperate, subtropical or humid tropical climates.72
The disease is benign and asymptomatic in immunocompetent individuals, but can progress to acute lung disease, and the severity depends on immune status, time of exposure and virulence of the strain.73 In the pulmonary alveoli, microconidia develop into yeast and are phagocytosed by macrophages through the CR3 receptor. However, Histoplasma capsulatum multiplies and induces apoptosis to spread to other cells.74 Neutrophils phagocytize opsonized yeasts through their CR1 and CR3 receptors, while non-opsonized yeasts are recognized by CD18 with the release of NETs.75 Components of azurophilic granules, such as BPI and cathepsin G inhibit yeast growth.8,76
Protection depends on cellular immunity; however, IL-17A production promotes granulopoiesis, production and recruitment of neutrophils to the site of infection.3,4,77 Study of neutrophil subcellular fractions has shown that yeasts promote NETs release and reduce their viability.78
Therapeutic strategies in COVID-19
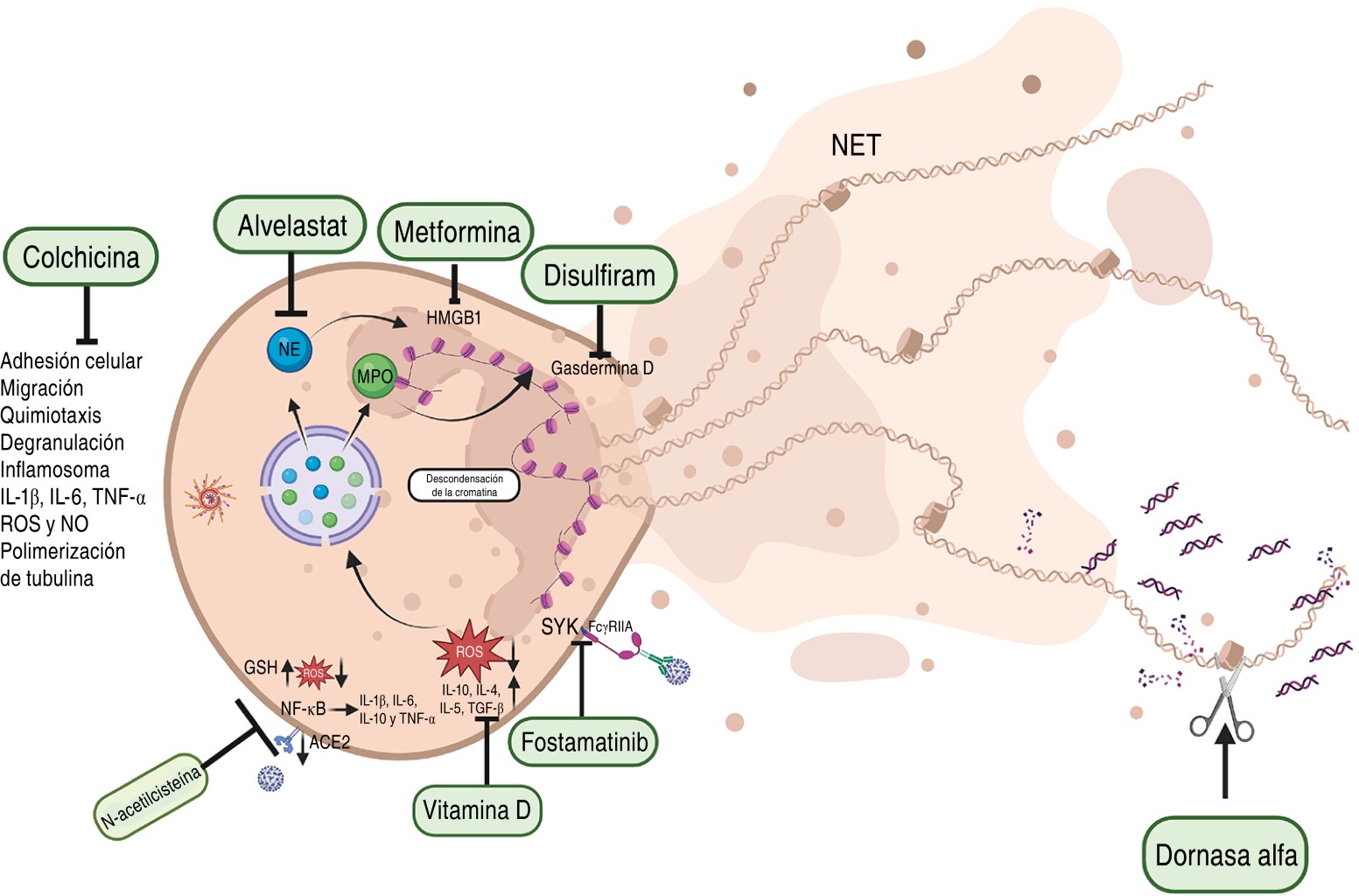
Mortality of patients with COVID-19 (with mechanical ventilation) was 24-53%, in part, due to the interference of mucopurulent secretions in ventilation. Neutrophil NETs contribute to the viscosity of secretions and are also present in serum.
There are almost 100 clinical studies involving the use of drugs to inhibit NET formation although only 19 have been completed.41 Studies have shown that the use of aerosolized dornase alfa (Pulmozyme, human recombinant DNAsa 1 and mucolytic) and albuterol decrease the viscosity of secretions by degrading DNA, improving oxygenation and reducing respiratory support in patients.79-82 In addition, complications are due to the presence of antigen-antibody complexes in the plasma, which interact with FcγRIIA receptors (CD32a) on the neutrophil membrane and favor the formation of NETs. However, fostamatinib is a drug that inhibits the Spleen Tyrosine Kinase (SYK) activation pathway associated with these receptors, reducing the formation of NETs.83 Also, there are controlled studies in COVID-19 patients using colchicine, since it interferes in inflammatory pathways inhibiting neutrophil adhesion, mobilization, degranulation and chemotaxis. It decreases cell adhesion molecules, consequently, it reduces migration and interaction of neutrophils with endothelial cells and their recruitment to the site of inflammation. It also prevents microtubule polymerization by inhibiting the formation of the NLRP3 inflammasome by reducing the production of IL-1β which prevents the production of IL-6 and TNF-α, and also inhibits the production of ROS and Nitric Oxide (NO).84,85
Additionally, drugs have been proposed to block NET components such as alvelestat which inhibits NE involved in inflammation,86 metformin which binds to HMGB1 (High mobility group box 1) inhibiting inflammation,87 disulfiram which binds to gasdermin D inhibiting its ability to cause membrane pores and promote NET formation in COVID-19 patients reducing inflammation and tissue damage.88
The production of ROS is critical as it favors the production of NETs, and the use of N-acetylcysteine, which has an antioxidant function favoring the production of reduced glutathione that decreases ROS; and an anti-inflammatory effect by preventing the binding of the SARS-CoV-2 virus to the ACE2 receptor and inhibits the activation of the transcriptional factor NF-κB reducing the production of inflammatory cytokines, have been proposed.89 Vitamin D has been proposed for use because it has anti-inflammatory actions by increasing the production of IL-10, IL-4, IL-5, and TGF-β, and because through antioxidant mechanisms it reduces oxidative stress and ROS production (Figure 4).90
Conclusions
Neutrophils are important in host defense; however, in viral infections such as COVID-19 they are associated with inflammatory events and thrombosis. The knowledge of their mechanisms of action has allowed proposing therapeutic alternatives that can provide a better prognosis to patients with COVID-19. While in tuberculosis and histoplasmosis they have limited participation in the resolution of the disease.
AFILIACIONES
1Medical College, Universidad Nacional Autónoma de México, Mexico City 2Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 3 Universidad Autónoma del Estado de Hidalgo, Mexico. 4Both authors contributed equally to the writing of the article.Conflict of interests: the authors declare that they have no conflict of interests.
REFERENCES
Jacobo-Delgado YM, Torres-Juarez F, Rodríguez-Carlos A, Santos-Mena A, Enciso-Moreno JE, Rivas-Santiago C, et al. Retinoic acid induces antimicrobial peptides and cytokines leading to Mycobacterium tuberculosis elimination in airway epithelial cells. Peptides. 2021;142: 170580. Available from: https://pubmed.ncbi.nlm.nih.gov/34033876/
Pitangui N de S, Sardi J de CO, Voltan AR, dos Santos CT, da Silva J de F, da Silva RAM, et al. An intracellular arrangement of Histoplasma capsulatum yeast-aggregates generates nuclear damage to the cultured murine alveolar macrophages. Front Microbiol. 2016;6:1526. Available from: https://pubmed.ncbi.nlm.nih.gov/26793172/
|
Table 1: Cytokines and chemokines secreted by neutrophils. |
|||
|
|
Function |
Target cell |
References |
|
Cytokines |
|||
|
IL-1α |
- Proinflammatory effect - Promotes proliferation and differentiation - Endogenous pyrogen |
T and B lymphocytes, MN, eosinophils, DC and fibroblasts |
27,30 |
|
IL-1β |
- Increases differentiation and IL-9, RORγt and IRF4 expression |
Subpopulations of T lymphocyte subpopulations: TH9 and TH17 |
27,30 |
|
IL-6 |
- Promotes inflammation - Hematopoiesis - Differentiation |
T and B lymphocytes |
27,30 |
|
IL-17 |
- Proinflammatory effect - Increases production of IL-1, IL-6, TNF-α, G-CSF, GM-CSF and chemokines that attract MN and neutrophils |
Endothelial cells, epithelial cells and fibroblasts |
27,30 |
|
IL-18 |
- Promotes TH1 lymphocyte differentiation - Induces IFN-γ production by T lymphocytes - Increases cytotoxic activity of NK lymphocytes |
T lymphocytes and NK cells |
30 |
|
TNF-α |
- Regulates the growth and differentiation of various cell types - Promotes angiogenesis, bone resorption and thrombotic processes - Suppresses lipogenic metabolism |
Neutrophils, macrophages, fibroblasts and T and B lymphocytes |
27,30 |
|
MIF |
- Promotes activation - Inhibits macrophage migration |
Macrophages |
30 |
|
IL-1 Ra |
- Anti-inflammatory activity - IL-1 antagonist, blocking its binding to the receptor, preventing a proinflammatory response |
MN, lymphocytes, fibroblasts and endothelial cells |
31 |
|
TGF-β |
- Anti-inflammatory effect - Inhibits growth, differentiation and functions of various cell types - Promotes angiogenesis and tissue repair - Stimulates the production of IgA antibodies |
T and B lymphocytes, MN, macrophages and fibroblasts |
27,30 |
|
IL-22 |
- Proinflammatory and anti-inflammatory effect - Stimulates transcription of protein genes with microbicidal activity |
Keratinocytes |
27,30 |
|
IL-23 |
- Promotes differentiation - Induces IL-17A and IL-17B synthesis |
TH17 lymphocytes |
27,30 |
|
G-CSF |
- Growth and differentiation of neutrophil precursors |
Neutrophils |
27,30 |
|
Chemokines |
|||
|
CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL8 (IL-8) |
- Proinflammatory activity - Neutrophil recruitment |
Neutrophils |
18,32 |
|
CXCL4 |
- Proinflammatory activity - Platelet aggregation |
Platelets |
27 |
|
CXCL9, CXCL10, CXCL11 |
- Proinflammatory activity - Recruitment of effector T-lymphocytes |
Effector T lymphocytes |
23,27 |
|
CCL2 |
- Proinflammatory activity - Leukocyte recruitment |
MN and basophils |
32 |
|
CCL3, CCL4 |
- Proinflammatory activity - Leukocyte recruitment - T-lymphocyte-DC interaction |
Macrophages, NK lymphocytes, T-lymphocytes and DCs |
27,33 |
|
CCL17, CCL22 |
- Migration and activation |
TH2 lymphocytes, regulatory T lymphocytes and basophils. |
27,33 |
|
CCL18 |
- Activation |
Lymphocytes TH2 |
33 |
|
CCL19 |
- Migration to lymph nodes |
DC and T lymphocytes |
22,32 |
|
CCL20 |
- Migration to intestinal lymphoid tissue |
TH17 lymphocytes, B lymphocytes and DCs |
33 |
|
CCL23 |
- Chemoattractive activity |
T lymphocytes, MN and neutrophils |
34 |
|
DC = dendritic cell. G-CSF = granulocyte-stimulating factor. GM-CSF = granulocyte-monocyte-stimulating factor. IFN = interferon. IgA = immunoglobulin A. IL = interleukin. IRF4 = interferon regulatory factor 4. Reg T lymphocytes = regulatory T lymphocytes. MIF = macrophage migration inhibitory factor. MN = monocyte. NK = natural killers. RORγt = retinoid orphan receptor gamma t. TGF-β = transforming growth factor-beta. Th = T helper lymphocyte. TNF = tumor necrosis factor. |
|||
|
Table 2: Toxic effects of NETs on lung epithelium and epithelial cells. |
|
|
NET components |
Cytotoxic effect |
|
DNA fibers |
Diffuse alveolar damage and hemorrhage |
|
Histones |
Increase the permeability of the endothelium |
|
NE |
Destruction of the cytoskeleton of endothelial cells. Affects the integrity of the alveolar barrier. Associated with inflammation and thrombosis by the release of proinflammatory cytokines. |
|
MPO |
Involved in epithelial cell apoptosis by the release of ROS |
|
DNA = deoxyribonucleic acid. MPO = myeloperoxidase. NE = neutrophil elastase. NET = neutrophil extracellular traps. |
|


