Benz[a]Anthracene exposure from e-cigarettes and its association with lung carcinogenesis. Descriptive review
Valencia-Cervantes, Jesús1,2; Sierra-Vargas, Martha Patricia1
Valencia-Cervantes, Jesús1,2; Sierra-Vargas, Martha Patricia1
ABSTRACT
Smoking is associated with a variety of adverse health effects, including cardiovascular and respiratory diseases, stroke, coronary heart disease, and cancer. Despite this, its use is widely prevalent throughout the world. In the past two decades, electronic cigarettes have emerged as an alternative to the use of conventional cigarettes. Nevertheless, the use of electronic cigarettes is not without adverse effects, particularly among adolescents and young adults, where respiratory, cardiovascular, and neurological damage has been demonstrated. However, further research is necessary to identify any potential association between electronic cigarette use and cancer incidence. Electronic cigarettes produce an aerosol upon heating e-liquids, which consist of mixtures of various compounds. These include particulate matter, nicotine, propylene glycol, glycerin, ethylene glycol, vitamin E, reactive oxygen species, heavy metals, volatile organic compounds, and polycyclic aromatic hydrocarbons, including Benz[a]Anthracene, which has the capacity to activate the aryl hydrocarbon receptor, that modulates the expression and activity of cytochrome P450 enzymes, thereby promoting the bioactivation of these compounds and the subsequent processes of mutagenesis and carcinogenesis. This review describes the potential risk of Benz[a]Anthracene content in electronic cigarettes, its activation-signaling pathway, and its association with lung carcinogenesis.KEYWORDS
electronic cigarettes, lung cancer, polycyclic aromatic hydrocarbons, cytochrome P450.Introduction
The global number of tobacco users is estimated to be 1.3 billion, representing a significant public health concern worldwide. The Global Burden of Disease report indicates that 7 million individuals use tobacco, while approximately 1.3 million non-smokers are exposed to second-hand smoke.1 Smoking increases the risk of developing various health issues, including cardiovascular and respiratory diseases, stroke, coronary heart disease, and certain types of cancer.2 Despite being marketed as a substitute for smoking, electronic cigarettes (e-cigs) can produce potentially harmful vapors. Several studies have demonstrated that both e-cigs aerosols and traditional tobacco products have detrimental effects.3 The number of people who use e-cigs (vapers) reflects trends in nicotine consumption and is expected to reach 82 million in 2021, up from the previous estimate of 58 million in 2018. In the future, the displacement of smokers in low- and middle-income countries, where 80% of the total smoking population resides, is projected to have the most significant impact.4,5 The prevalence of vapers has consistently increased among individuals aged 21-24 years. However, the health risks associated with the use of e-cigs at an early age are currently unknown.6 As reported by Dahal et al., the concurrent use of e-cigs and conventional smoking was the most prevalent among older individuals. The females (44.7%) were more likely than the males (39.8%) to have initiated e-cig use at an earlier age. The majority of the older population (> 45 years) used e-cigs for the cessation of smoking (74.1%), whereas the majority of the younger population used them for recreational (50.2%).7 E-liquids (e-liqs) when are vaporized, they release compounds that may be equal to or different from the parent compound and may be more dangerous due to their physicochemical characteristics.8-10 This review evaluates the potential impact of polycyclic aromatic hydrocarbons (PAH) compounds, particularly benz[a]anthracene (B[a]A) found in e-cigs, on the lung carcinogenic process. The information consulted was obtained using the keywords "electronic cigarettes", "polycyclic aromatic hydrocarbons", "aryl hydrocarbon receptor", and "lung cancer" in PubMed, Science Direct, Springer Link, and Science.gov. The works that employed in vitro and in vivo models were considered, regardless of the species used in the study.
Chemical composition of e-liqs and e-vapors
E-cigs consist of three primary components: an e-liq chamber, an atomizer, and a battery. The atomizer contains a heating coil activated by the battery, which heats the e-liq in the cartridge. This heating process produces an inhalable e-vapors (e-vaps) that resembles the smoke produced by traditional cigarettes. When the user inhales, a sensor is triggered, initiating the heating process of the e-liq in the cartridge. E-liqs are a crucial component of the vaping system.9,11 When burned, cigarettes generate more than 7,000 chemicals, including at least 69 known to cause cancer or toxicity, from approximately 600 ingredients.2 In contrast, e-cigs do not contain tobacco, but the temperature at which e-vaps is produced contributes to the formation of several compounds.11 Kuehl et al., demonstrated that flavoring compounds undergo thermal degradation at both low (250 °C) and high (750 °C) temperatures. This process results in the formation of e-vaps containing ~300 compounds, including volatile organic compounds (VOCs), carbonyls (aldehydes and ketones), PAH, particulate matter, nicotine, propylene glycol, glycerol, ethylene glycol, vitamin E, flavors, reactive oxygen species (ROS), and heavy metals (Pb, Ni, and Cr).8-10,12 E-liqs flavors used to simulate or enhance the taste of e-cigs include compounds such as piperidine, butanoic acid, ethyl maltol, vanillin, 1-butanol 3-methyl acetate, and ethyl acetate. These compounds are classified as Generally Recognized as Safe due to their longstanding use in food production, but are limited to ingestion and do not extend to inhalation.13 Nawi et al., analyzed seventy-two e-liqs from over 60 brands to determine the principal presence of VOC, flavors, nicotine, propylene glycol, and glycerin in 75%, in addition, the analysis of e-liqs identified 116 compounds while e-vaps contained 275 compounds. Forty-two compounds were found in both e-liqs and e-vaps, seven were found only in e-liqs and thirty-eight were found only in e-vaps.11 Also, Czoli et al., identified a total of 119 flavoring chemicals, including benzaldehyde (21.7%), vanillin (21.7%), and benzyl alcohol (19.9%). Other chemicals, such as 2-acetyl pyrazine, acrolein, cinnamaldehyde, diacetyl, toluene, diacetin, acetone, and isopropyl alcohol, were also detected but at lower frequencies. It also found that tobacco-specific nitrosamines were present, which are potential carcinogens such as N-nitrosonornicotine at 69.8% and nicotine-derived nitrosamine ketone at 42.1%.14 Beauval et al., identified trace elements (Cd, Cr, and Sb) in six e-liqs and their corresponding e-vaps. Some e-liqs samples contained pesticides such as chlorpyrifos ethyl and trifluralin, but they were not detected in the e-vaps. The major PAH found in e-liqs and e-vaps were naphthalene, phenanthrene, formaldehyde, and acetaldehyde.15
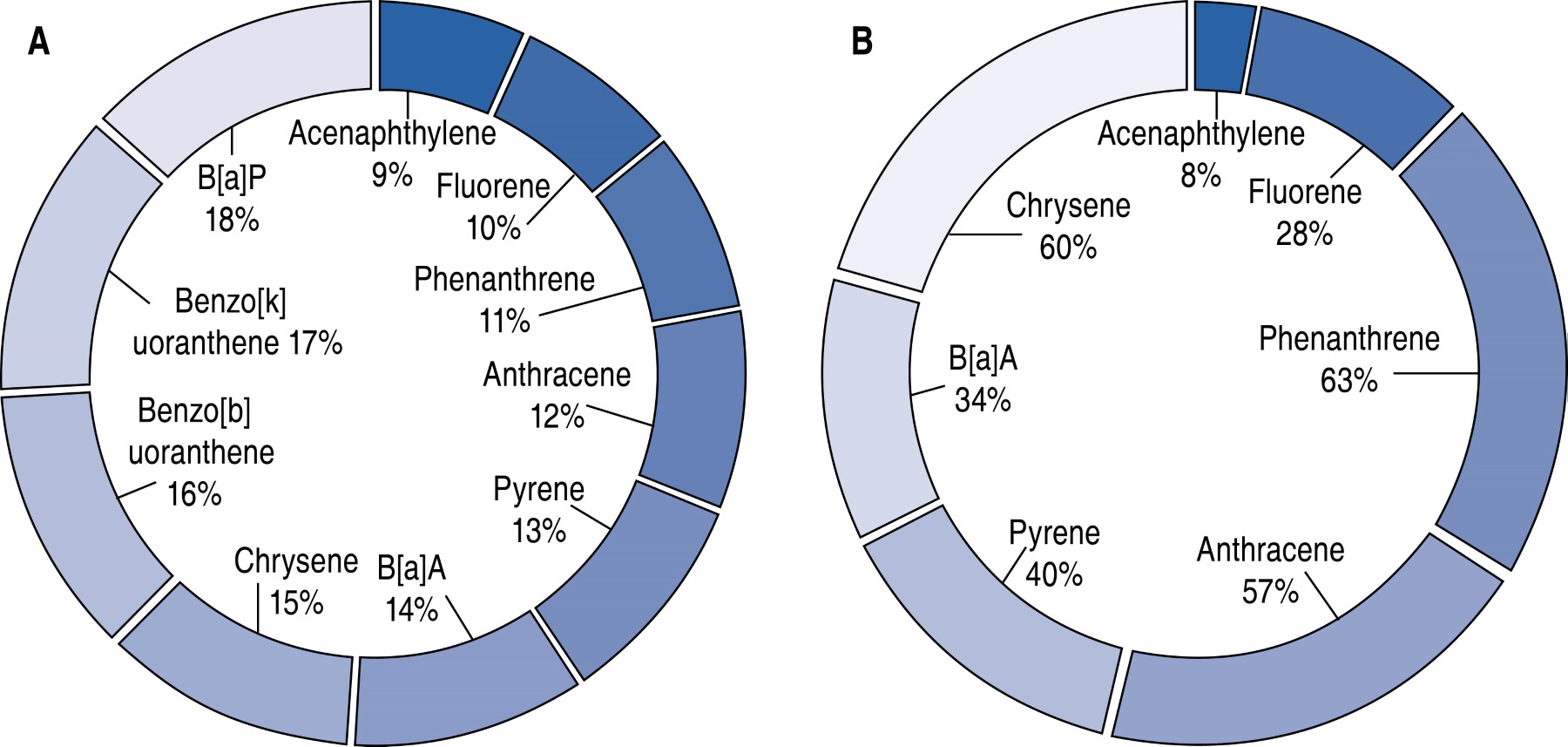
Kosarac et al., analyzed 825 e-liqs and identified 1,507 compounds, with the total chemicals detected exceeding 14,000, including nicotine, propylene glycol, glycerol, β-Nicotyrine, and nicotine oxidation products.16 Finally, Larcombe et al, found in sixty-five samples of e-liqs and e-vaps containing excipients, solvents, flavorings, nicotine, and PAH such as B[a]P, B[a]A, acenaphthylene, acenaphthene, anthracene (ANT), chrysene, fluorene, fluoranthene, phenanthrene, naphthalene (NAP), and pyrene. It is important to note that B[a]P and B[a]A have mutagenic and carcinogenic properties. Although low levels of PAH in e-cigs and e-vaps products may not individually pose a risk of causing disease, their presence as a mixture may potentiate their effects and lead to adverse health outcomes.17 Compounds such as B[a]A, found in e-cigs and belong to the PAH group, are of great concern due to their ability to cause mutagenesis and carcinogenesis.18 The processes are initiated by activating the aryl hydrocarbon receptor (AHR) and cytochrome P450 (CYP) enzymes, which play a significant role in the bioactivation and metabolism of carcinogenic agents.19 Figure 1A and 1B illustrates the classification of compounds and the PAH identified in e-cigs and e-vaps.
Activation of the AHR
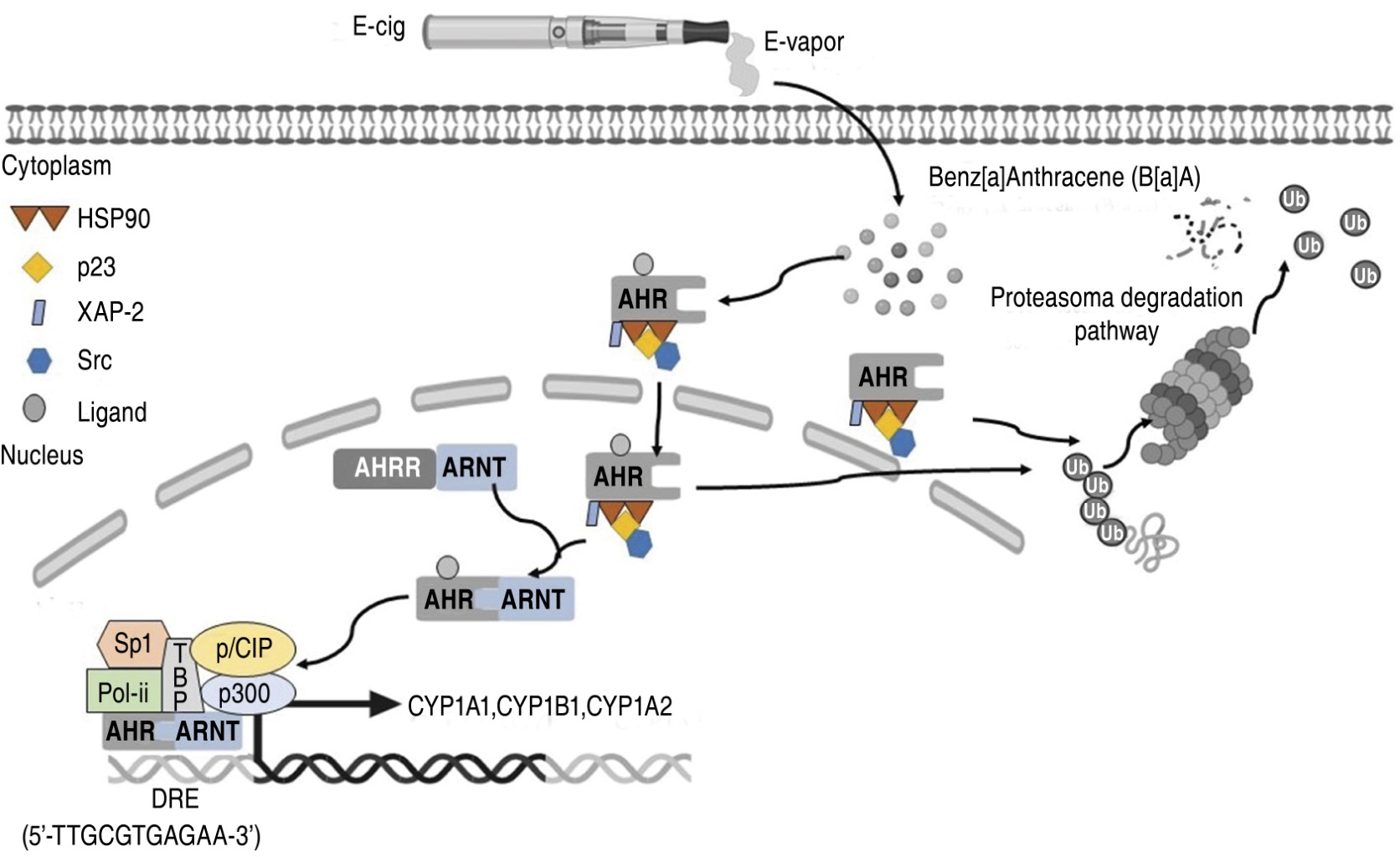
PAH are a group of chemicals that contain two or more fused aromatic rings. Sixteen are priority pollutants due to their high occurrence and toxicity. Among them, B[a]P, B[a]A, benzo[b]fluoranthene, benzo[j]fluoranthene, and benzo[k]fluoranthene have been found to have genotoxic, mutagenic, and carcinogenic effects.18 The toxicity of PAH is associated with reactive electrophilic metabolite formation and the activation of cellular receptors, such as the AHR. The AHR is a transcription factor that belongs to the basic helix-loop-helix-PER-ARNT-SIM (bHLH-PAS) subgroup of the bHLH superfamily and is exclusively activated by ligands. Activation of the AHR pathway leads to the transcription of several CYP genes, which play a significant role in the bioactivation and metabolism of carcinogenic agents.19 Figure 2 shows how PAH, such as B[a]A, activate the AHR pathway by regulating the expression of CYP involved in their metabolism. Many compounds bind to the AHR with high affinity, causing ligand-dependent changes in its activity. The AHR is an attractive target for small molecule manipulation due to its potential for strong agonist activity. Examples of compounds that fall under this category include persistent planar halogenated polycyclic hydrocarbons (2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,7,8-tetrachlorodibenzofuran, B[a]A, B[a]P, 3-methylcholanthrene, and β-naphthoflavone), flavonoids (quercetin, apigenin, and kaempferol) and cruciferous vegetables (curcumin, indole-3-carbinol), are considered ligands.19 Certain ligands, including resveratrol and galangin, can display agonist and antagonist activities.20
Formation of a 3,4-diol-1,2-epoxide metabolite of B[a]A
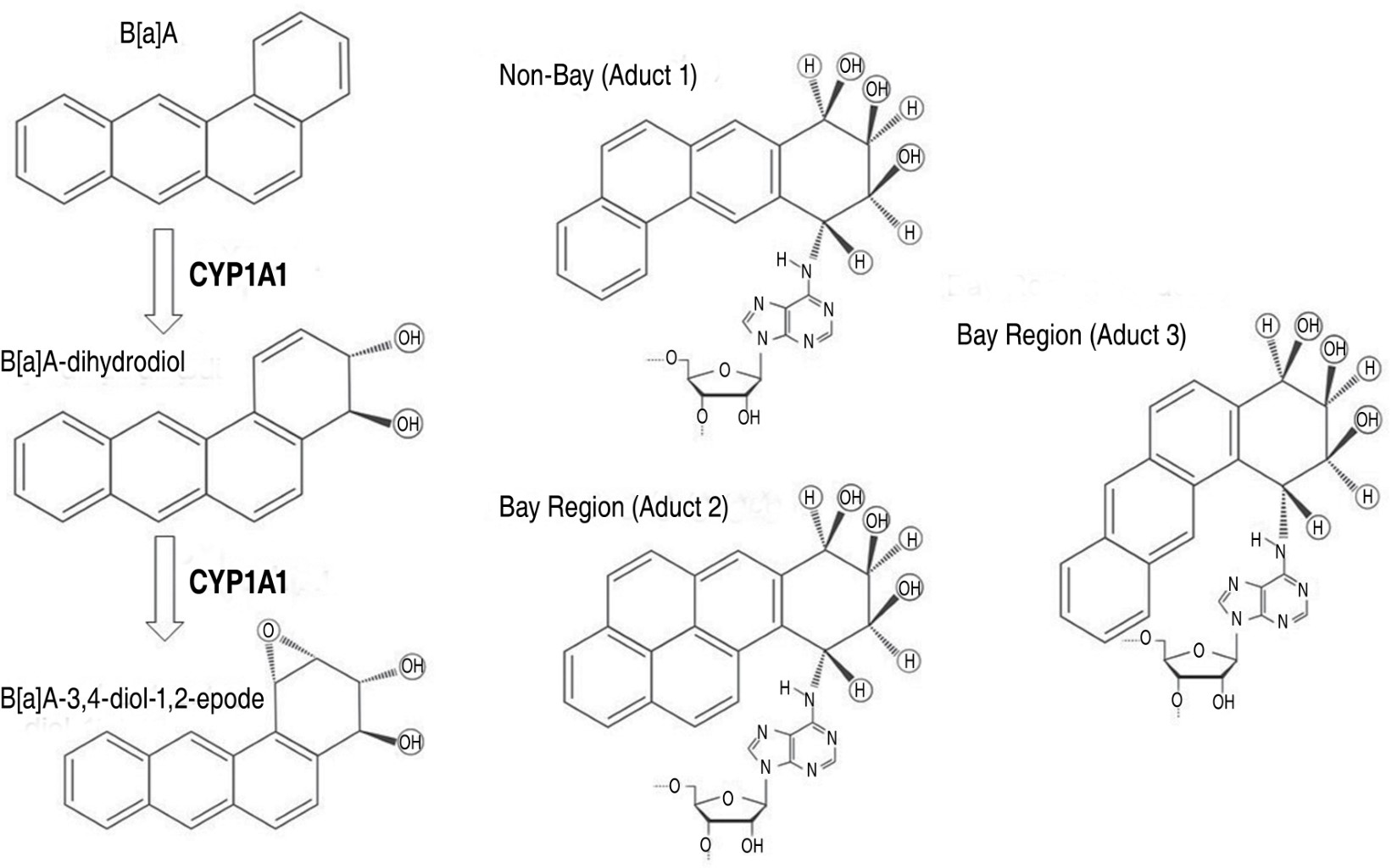
B[a]A is mainly produced by burning fossil fuels and is found in tobacco smoke. It can bind to suspended particulate matter and accumulate in aquatic organisms. It can also adsorb solid particles in soil, which extends its half-life to 261 days. Its atmospheric half-life is about 7.7 hours due to photochemical degradation.21 Figure 3 shows the principal metabolic pathway for the carcinogenic mechanisms of B[a]A by CYP1A1. The stereoisomeric diol epoxides found in the bay-region exhibit significant tumor-initiating activity. It is widely acknowledged that diol epoxides formed from the oxidative metabolism of PAH compounds in the bay-region are more mutagenic than their non-bay counterparts. Furthermore, the resulting adduct structures impede DNA replication and repair, leading to increased mutations. Mutagenicity was observed in bay-region B[a]A adducts when tested in a repair-deficient prokaryotic in vivo replication system. However, the corresponding non-bay region adducts of B[a]A were easily bypassed by Escherichia coli replication complexes and were not mutagenic.22 Song et al., discovered that exposing hepatoblastoma HepG2 cells to B[a]A led to an increase in gene and protein expression for CYP1A1 and CYP1B1. Furthermore, it was found that B[a]A-3,4-diol-1,2-epoxide has more potent cytotoxic and genotoxic effects than higher doses of B[a]A.23 The results also suggest that treatment with trans-3,4-dihydroxy-3,4-dihydro-B[a]A in mice resulted in a 24% incidence of malignant lymphoma, while only 4% of the subjects treated with B[a]A developed the same condition. Moreover, it was found that trans-3,4-dihydroxy-3,4-dihydro-B[a]A induced about 35 times more lung adenomas than B[a]A.24 Additionally, Wood et al, reported that B[a]A-3,4-dihydrodiol was 10 times more mutagenic than B[a]A in Salmonella typhimurium. Furthermore, it was discovered that trans-dihydrodiol-B[a]A can cause skin tumors in female CD-1 mice. The mutagenic activity of the two diastereomeric 1,2-epoxides of trans-3,4-dihydrodiol-B[a]A is significantly higher in Salmonella typhimurium and strain V79-6 of Chinese hamster lung cells than the diastereomeric pairs of B[a]A-8,9-diol-10,11-epoxides or B[a]A-10,11-diol-8,9-epoxides.25,26 Additionally, male mice exposed to the (+)-diol-epoxide-2 isomer showed a significant incidence of hepatic tumors. The results indicate that bay-region epoxides of unsubstituted PAH are reactive forms of these carcinogenic compounds.25-27 According Levin et al, reported a significant occurrence of tumors caused by two isomers of bay-region diol-epoxides. The (+)-diol-epoxide-2 isomer induced a 100% incidence of lung tumors in mouse models, whereas the (+)-diol-epoxide-1 isomer produced a 31% incidence.28
Regulation of cellular functions by the AHR and CYP1 family in the lung
PAH are lipophilic compounds that can passively diffuse across cell membranes when inhaled. Enzymes, including CYP, metabolize PAH to form phenols, catechols, and quinones. These compounds can react with DNA, forming adducts, such as diol-epoxides, radical cations, or reactive and redox-active quinones.29 The activation of AHR increased in human bronchial epithelial cells, associated with pulmonary diseases, such as asthma and chronic obstructive pulmonary disease.30 Matsumoto et al, demonstrated that AHR plays a significant role in carcinogenesis induced by airborne particulate extract in mice, as well as in the activation of carcinogenic PAH by CYP1A1.31 Additionally, Lin et al., found that the protein expressions of AHR were higher in two out of four adenocarcinoma A549 cells compared to bronchial epithelial BEAS-2B cells and squamous carcinoma cells.32 The metabolism of PAH involves three functional genes in two subfamilies of the CYP1 family. The catalytic activities of CYP1 enzymes overlap and include hydroxylation and other oxidative transformations of B[a]P, B[a]A, and other aromatic substances.33 CYP are responsible for processing 75% of the xenobiotics in humans.34 Shimada et al., found that tumor formation rates were lower in CYP1B1 gene-knockout mice treated with 7,12-dimethyl-B[a]A and dibenzo[a,l]pyrene compared to wild-type mice.35 Additionally, PAH and polyhalogenated hydrocarbons, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin, induce gene expression of CYP1A1 and CYP1B1 via the AHR. Addition the effects of three PAH (5-methylchrysene, B[a]P) and B[a]A) inhibited the metabolic activation of 5-methylchrysene-1,2-diol, B[a]P-7,8-diol, dibenzo[a,l]pyrene-11,12-diol, and 2-amino-3,5-dimethylimidazo[4,5-f]quinolone to genotoxic metabolites catalyzed by CYP isoforms 1A1, 1B1, and 1A2 in Salmonella typhimurium.36 The distribution of CYP1 enzymes was detected in bronchial and bronchiolar epithelium, Clara cells, alveolar lining cells, and alveolar macrophages. CYP1A1 expression is limited to the epithelium of the peripheral airways in the lungs of smokers and does not extend to bronchial epithelium larger than > 1 mm in diameter. Furthermore, alveolar macrophages do not express CYP1A1.37
Diseases of the respiratory tract associated with B[a]A
PAH mixtures have short-term (acute) effects that cause skin irritation and inflammation; ANT, B[a]P, and NAP are direct skin irritants. Additionally, ANT and B[a]P have been reported to be skin sensitizers, causing an allergic reaction in animal models and humans. Long-term (chronic) effects include decreased immune function, cataracts, and damage to the kidneys, liver, and lungs, resulting in breathing problems, asthma-like symptoms, immunotoxicity, neurodevelopmental abnormalities, thyroid dysfunction, and disruption of steroid hormones and reproductive functions.38,39 The American Cancer Society is closely monitoring research on the health effects and symptoms of newly introduced tobacco products with PAH, such as coughing, shortness of breath, chest pain, nausea, vomiting, or other related.40
Analysis of epithelial cells from biopsy samples revealed approximately 300 differentially expressed proteins in the airways of smokers and vapers. Among these, seventy-eight proteins showed alterations in both groups, while 113 were unique to vapers. The study found that vapers exhibited increased levels of CYP1B1. Additionally, the research showed that e-liqs rapidly enter the cells. The study also observed that propylene glycol and vegetable glycerin decrease membrane fluidity and hinder protein diffusion, suggests that long-term vaping may have significant biological effects on the lungs, which could lead to the development of chronic lung disease.41 E-vaps on human bronchial epithelial HBEC cells resulted in 546 differentially expressed genes among the e-cigs, tobacco cigarettes, and air-exposed groups. Moreover, the exposure to e-cigs activated genes associated with oxidative and xenobiotic stress pathways and increased the production of ROS.42 E-cigs or vaping use-associated lung injury (EVALI) can cause severe lung damage and inflammation. The vitamin E acetate is the most recognized agent associated with e-cig use with EVALI, as evidenced by the presence of vitamin E acetate in bronchoalveolar lavage fluid samples of 48/51 patients with EVALI, in contrast to the absence of vitamin E acetate in the samples obtained from the healthy control.43 Among hospitalized patients with EVALI, several chronic conditions were prevalent, including asthma, chronic obstructive pulmonary disease, cardiac disease, and any mental health condition.44 Nicotinic acetylcholine receptors regulate the cystic fibrosis transmembrane conductance (CFTR) in the airways. Inhaling nicotine can adversely affect these receptors, leading to impaired CFTR function. This decrease in CFTR function has been associated with the progression of asthma, hypertension, and chronic obstructive pulmonary disease.9,45-47
Lung cancer
Genetic susceptibility and multiple factors contribute to the activation and elimination of PAH, which can lead to lung cancer development. Exposure to PAH for extended periods was associated with tumor formation in various organs, such as the lungs, prostate, bladder, colon, stomach, breast, and oral cavity.48 Staudt et al., discovered that after inhaling e-cigs containing nicotine, ten non-smokers experienced alterations in the transcriptomes of small airway epithelium and alveolar macrophages and elevated levels of plasma endothelial microparticles, disrupt normal human lung homeostasis.49 Cioroiu et al., assessed the levels of PAH in the lungs of thirty-one Romanian patients with lung cancer, identified fifteen PAH, including B[a]P, B[a]A, fluoranthene, benzo[b]fluoranthrene, and benzo[k]fluoranthrene.50 This compelling evidence that PAH are etiological factors in human lung cancer. Tang et al., demonstrated that exposure to e-cigs for fifty-four weeks in FVB/N mice resulted in the development of lung adenocarcinomas (22.5%) and bladder urothelial hyperplasia (57.5%). This indicates that e-cigs damage DNA in both lung and bladder tissues and inhibit DNA repair in the lungs.51 Canistro et al., found that e-vaps have co-mutagenic and cancer-initiating effects on male Sprague Dawley rats, discovered that e-cigs enhance the activity of phase-I carcinogen-bioactivation enzymes, leading to increased production of ROS and DNA oxidation, resulting in 8-hydroxy-2'-deoxyguanosine.47 Lee et al., investigated the impact of nitrosamines from e-cigs on DNA damage in multiple organs of FVBN mice and discovered the presence of mutagenic O6-methyldeoxyguanosines and γ-hydroxy-1,N2-propano-deoxyguanosines in the lung, bladder, and heart and found a significant reduction in DNA-repair activity and repair proteins in the lung.52 Zahedi et al, showed that exposure of human adenocarcinoma A549 cells to menthol or tobacco-flavored e-liqs or e-vaps induced an Epithelial-to-Mesenchymal Transition (EMT). This transition was characterized by the acquisition of a fibroblast-like morphology, loss of cell-to-cell junctions, internalization of E-cadherin, increased motility, and upregulation of other EMT markers in the oral epithelium of both vapers and smokers. Molecular pathway showed that cancer was the primary disease associated with the deregulated genes in both vapers and smokers, with a prevalence of ~62% and ~79%, respectively.53 According to Tommasi et al., the Wnt/Ca²⁺ pathway was the most affected in vapers, while the integrin signaling pathway was in smokers. The GTPases signaling pathway was identified as the top disrupted pathway.54 It has been demonstrated that menthol has direct toxic effects on the lung and other tissues and potential oncogenic effects.55 Other substances found in e-cigarettes include formaldehyde, toluene, acetaldehyde, and acrolein, as well as heavy metals that are associated with the incidence of cancer of the head, neck, bladder, and breast. However, further research is required to substantiate the direct relationship between these compounds and the incidence of a specific type of cancer.56
Conclusion
E-cigs, as an alternative to conventional cigarettes, do not prevent the occurrence of adverse effects. The e-vaps contain several chemical compounds, including PAH such as B[a]A, which is a potential carcinogen. When the e-vaps are generated, they release other compounds that are equally or more toxic than the parent compounds. The evidence indicates that B[a]A regulates the AHR signaling pathway, which in turn induces the expression of CYP genes that participate in the metabolism of this PAH, favoring the formation of active metabolites with carcinogenic capacity. This suggests that B[a]A may be an etiological factor in human lung cancer. Furthermore, the presence of low levels of PAH in e-cigs and e-vaps products may not individually pose a risk of causing disease. However, their combined presence may potentiate their effects and lead to adverse health outcomes. Currently, there is no official regulation of the chemical composition of e-liqs, making their control and sales difficult. Although e-cigs have gained popularity among young people since their appearance two decades ago, recent information warns about potential health risks associated with their use. Therefore, it is necessary to identify molecular targets and signaling pathways that can prevent the emergence of any disease.
AFILIACIONES
1Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Ciudad de México 2Consejo Nacional de Humanidades, Ciencias y Tecnologías, Ciudad de México.Acknowledgments: the authors gratefully acknowledge Estancias Posdoctorales por México 2022 (1), CONAHCYT.
Funding: the authors gratefully acknowledge the Proyecto de Ciencia Frontera 2019 (CF-MG-20191024195736984-840342).
Conflict of interests: the authors declare no interest conflicts.
Author contributions: the manuscript was developed through a collaborative process by JVC and MPSV. The same authors were also responsible for the collection and analysis of data for purposes of manuscript revision. The authors have read and approved the final version of the manuscript.
REFERENCES
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK179276/
Li Z, Kim HY, Tamura PJ, Harris CM, Harris TM, Stone MP. Role of a polycyclic aromatic hydrocarbon bay region ring in modulating DNA adduct structure: the non-bay region (8S,9R,10S, 11R)-N(6)-[11-(8,9,10,11-tetrahydro-8,9, 10-trihydroxybenz[a]anthracenyl)]-2'-deoxyadenosyl adduct in codon 61 of the human N-ras protooncogene. Biochemistry. 1999;38(45):14820-14832. doi: 10.1021/bi991607z.
Schier JG, Meiman JG, Layden J, Mikosz CA, VanFrank B, King BA, et al.; CDC 2019 Lung Injury Response Group. Severe pulmonary disease associated with Electronic-Cigarette-Product Use - Interim Guidance. MMWR Morb Mortal Wkly Rep. 2019;68(36):787-790. doi: 10.15585/mmwr.mm6836e2 Erratum in: MMWR Morb Mortal Wkly Rep. 2019;68(38):830. doi: 10.15585/mmwr.mm6838a4.
Tang MS, Wu XR, Lee HW, Xia Y, Deng FM, Moreira AL, et al. Electronic-cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice. Proc Natl Acad Sci U S A. 2019;116(43):21727-21731. doi: 10.1073/pnas.1911321116 Erratum in: Proc Natl Acad Sci U S A. 2019;116(45):22884. doi: 10.1073/pnas.1918000116.